Louis Rucker, Audrey Muck, Mark Broering, Patrick Muck
|
Abstract Venous stenting was introduced in the 1990s and has continued to evolve to become the first-line therapy for symptomatic iliofemoral venous outflow pathology. There are several dedicated venous stents available in addition to Boston Scientific’s Wallstent and Cook’s Z-Stent. Numerous studies from tertiary referral centres, as well as industry-sponsored trials, have demonstrated the safety and efficacy of these endovascular devices for non-thrombotic iliac vein (NIVL) and post-thrombotic syndrome (PTS) lesions. Patients presenting with acute deep venous thrombosis (aDVT) may also undergo stenting following thrombus removal. The standard of care for NIVL, PTS and aDVT patients has become venography and intravascular ultrasound, and if an underlying iliofemoral stenosis is identified, a stent is placed. There is a concern that inflammation may affect the results of stenting in the aDVT population. Although endovascular stenting for acute venous diseases appears promising and safe, there is a paucity of data on efficacy in aDVT patients. There are only two industry-sponsored trials and a few publications and presentations at academic society meetings to examine. This review assesses the available results for endovascular stenting for aDVT and PTS patients, but not for NIVL. Keywords: Stent, venous, deep venous thrombosis, iliocaval, femoral, iliac Disclosure: PM is on the advisory board for Medtronic and Boston Scientific, and has received payment for lectures at Penumbra and BD. All other authors have no conflicts of interest to declare. Received:Accepted:Published online: Citation:Vascular & Endovascular Review 2023;6:e03. DOI: https://doi.org/10.15420/ver.2022.07 Acknowledgements: The authors thank Hatton Research Institute, TriHealth Inc. (Cincinnati, OH, US) for research support and guidance. Correspondence Details: Patrick Muck, Division of Vascular Surgery, TriHealth – Good Samaritan Hospital, 375 Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly. |
Chronic venous disease can be due to deep, superficial or perforator venous insufficiency, including obstruction, stenosis or valvular reflux. Deep venous disease can be secondary agenesis, stenosis, obstruction or reflux. Venous stenosis and obstruction can be due to aDVT, PTS or non-thrombotic iliac vein lesions (NIVL) and/or reflux due to valvular insufficiency.6 A high percentage of limbs with iliac vein obstruction will also have reflux below the inguinal ligament, resulting in peripheral venous hypertension.7 These pathologies cause chronic venous hypertension, resulting in inflammatory changes to peripheral tissues. Clinically, this causes limb pain, oedema, lipodermatosclerotic changes and venous leg ulcerations. Over 25 million people worldwide are affected by deep venous obstruction. Symptomatic iliofemoral outflow obstruction is a substantial contributor to chronic venous insufficiency and is increasingly being treated through an endovascular approach.
Table 1: Currently Available Venous Stents
Venous stenting was introduced in the 1990s and has continued to evolve as a viable option for iliac venous pathology.7–10 Although endovascular treatment for venous diseases appears promising and safe, there is a need to improve our knowledge of optimal venous stent strategies.6 There are several dedicated venous stents available, in addition to Boston Scientific’s WALLSTENT and Cook’s Z-Stent (Table 1), for symptomatic iliofemoral outflow obstruction. These devices perform well for NIVL and PTS. Although endovascular stenting for acute venous diseases appears promising and safe, there is a paucity of data on efficacy in aDVT patients, with only two industry-sponsored trials and a few publications and presentations at academic society meetings to examine. Unfortunately, not every paper or academic society presentation used the same definitions of aDVT or PTS. In this review, we define aDVT as ≤4 weeks in duration and PTS as >4 weeks in duration. This review assesses the available results for endovascular stenting for aDVT and PTS patients, but not for NIVL.
Venous Pathophysiology
Three major types of iliocaval venous obstruction are recognised: NIVL, aDVT and PTS iliac vein stenosis.7,8,10 NIVL vessels tend to harbour focal short lesions secondary to compression by an adjacent artery, other extrinsic lesions or the inguinal ligament. Cockett et al. originally described the first NIVL in 1900, and the term ‘May–Thurner syndrome’ was later used.11
Today, a diverse spectrum of non-thrombotic iliac vein lesions are identified using venograms and IVUS. These imaging techniques have confirmed that iliac vein compression posterior to the crossing right common iliac artery is present in as many as two-thirds of the general population.7 Increased use of IVUS has shown venous stenoses to be present at more diverse locations in the pelvic venous anatomy and that it affects a much broader demographic than the narrow band recognised by Cockett et al. The Venogram versus IVUS for Diagnosing Iliac vein Obstruction (VIDIO) trial concluded that IVUS was more sensitive and specific in detecting venous stenoses >50% than multiplanar venography.12 The available data suggest NIVLs to be focal lesions.
Post-thrombotic syndrome is the lifelong sequela of a DVT. The result is pain, swelling and skin changes in up to 50% of patients treated with anticoagulation alone. In the acute phase, the vessel has a thrombus with mild surrounding vessel wall inflammation. Research has shown that a vein undergoes a fibrotic process secondary to intense inflammation following DVT. Studies have also shown that the vein may undergo thickening as it responds to thrombus resolution with inflammation and increased biomarkers.13 As a result, blood flow and haemodynamics will be negatively altered. In its most severe form, the vein will end up with a long-segment chronic occlusion. These fibrotic changes are considered the reason for decreased patency following venous stenting for PTS lesions as opposed to NIVLs.
Today, many devices have proven effective in treating patients with acute DVT. IVUS is recommended following thrombus removal to identify underlying iliofemoral venous outflow obstruction. Currently, there are limited data on the venous pathophysiology following thrombus removal. Inflammation likely occurs secondary to the venous thrombus, but there is no consensus on the short- and long-term effects. Research is needed to determine whether the aDVT vein reacts like an NIVL or PTS following thrombolysis or thrombectomy. There are also limited data on the long-term effects of endovascular stenting following thrombus removal for aDVT. Furthermore, the role of anticoagulation and inflammation management is not well studied either. Most key opinion leaders believe full anticoagulation is needed in this scenario of inflammation and thrombogenicity.
In a recent presentation at the 34th Annual Meeting of the American Venous Forum, Marston concluded that treatment with low molecular weight heparin (LMWH) should transition to a direct oral anticoagulant (DOAC) at 4 weeks.14 The data presented indicated better patency with LMWH in these acute thrombotic situations compared with DOACs.15
Indications for Stenting
Chronic venous disease, in general, is a non-lethal disease and the loss of a limb is a rarity. If symptoms resulting from iliocaval or iliofemoral stenoses are present, conservative treatment with compression is the initial treatment modality. This modality will fail in ≥50% patients because of inefficacy or, more often, non-compliance with compression regimens. Iliac vein stenting may be carefully considered after failure of conservative treatment. Patients are categorised based on the Clinical–Etiology–Anatomy–Pathophysiology (CEAP) system. In general, only patients with CEAP classes 3–6 are candidates for the correction of underlying venous lesions. A recent randomised study compared outcomes of medical treatment versus iliac vein stenting in patients with chronic venous diseases and found significant reductions in pain and the Venous Clinical Severity Score (VCSS), along with a significant improvement in quality of life following endovascular treatment.15 Despite a lack of prospective randomised trials for venous stenting patient populations, both the American Venous Forum/Society of Vascular Surgery and European Society of Vascular Surgery have guidelines on the management of chronic venous disease.16,17 Currently, percutaneous transluminal angioplasty and stenting are recommended as first-line treatments in patients with symptomatic iliofemoral venous outflow obstruction.9,16,17
Endovascular Stenting Technique
Arterial and venous haemodynamics differ. Perhaps the only analogy between arterial and venous stenting is the common vascular principles of inflow, outflow and conduit. A well-thought-out plan beginning with preprocedural imaging is a necessity. CT venography and duplex imaging are the most commonly used modalities. Popliteal, high posterior tibial, mid-thigh ipsilateral femoral vein or internal jugular vein access under ultrasound guidance is preferred. A large sheath, typically 8–10 Fr, is preferred for easy manipulation of inserted devices, larger if a Z-Stent is used. These approaches allow enough room for the sheath to deploy stents below the inguinal ligament if needed. IVUS examinations of the inferior vena cava (IVC), common iliac vein, external iliac vein, common femoral vein and profunda femoral vein are conducted to identify lesions and appropriate landing sites. IVUS is used to measure diameters and areas. The degree of stenosis is best calculated using the expected normal area for the location. Using the ipsilateral adjacent normal lumen as a reference is the method used the most. Most symptomatic limbs will have ≥50% area reduction, although some lesser lesions can be symptomatic in individual patients with PTS due to severe compliance changes. Recently Gagne et al. showed that a 61% reduction in diameter, not area, is correlated with symptom relief after stenting.18
Treating the entire diseased segment or lesion in continuity with landing sites clear of disease is essential for successful outcomes. The principles of inflow, outflow and conduit apply in venous therapies. A high-pressure balloon for pre- and post-dilation is necessary for venous lesions. Generally, the stent size is based on the proximal or distal normal-appearing vein. The diameter of this normal-appearing vein is measured, and most interventionalists then add 2 mm for dedicated venous stents, ensuring placement and no risk of migration. Therefore, stent coverage from normal to normal is necessary, and a longer stent rather than a shorter stent is preferred. Complete stent coverage means longer stents, which also equates to less chance of migration to the right heart if undersized. Limbs with post-thrombotic disease may require extension of the stent below the inguinal ligament into the common femoral vein. The stent end should remain above the orifice of the deep femoral vein, which provides adequate inflow in most instances to sustain the stent. Occasionally, the stent can be delivered into the deep femoral vein (via jugular, popliteal or direct deep femoral access) if its ostium is involved in the post-thrombotic process. Stent fractures and erosions have been rare with stents crossing the joint crease, and patency has been shown to be sustained with a dedicated venous stent.19
Available Venous Stents and Data on Patients with Acute Deep Venous Thrombosis and Post-thrombotic Syndrome
WALLSTENT
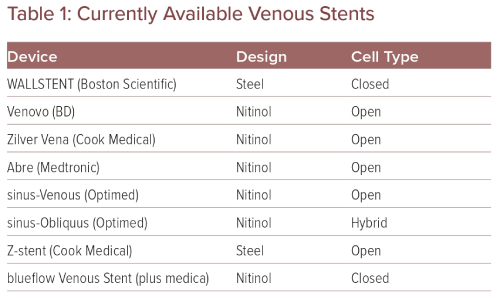
The WALLSTENT endoprosthesis from Boston Scientific has been the workhorse for venous stenting for several decades. Raju and Neglén’s early work is remarkable and highlights the WALLSTENT’s decades of benefit for patients with venous occlusive disease.8 The WALLSTENT is a closed-cell woven stent with a wide variety of lengths and diameters. The WALLSTENT has very good crush resistance and radial force, and performs quite well through the pelvic tortuosity because of its good flexibility. The short- and long-term outcomes are impressive, and several studies have shown its success and durability in terms of both patency and clinical outcomes.2–5,20,21 Because the WALLSTENT is a closed-cell stent, there is a concern it can impede flow from the contralateral iliac leading to contralateral iliac DVT.21 Successful treatment of an occluded IVC filter with two WALLSTENTs is shown in Figure 1.
Figure 1: WALLSTENT Placement Following Thrombectomy of Acute Inferior Vena Cava Filter Occlusion
There are studies on stenting for acute DVT that included the WALLSTENT in most cases. The meta-analysis by Razavi et al. includes a category for acute thrombotic studies.22 That review noted that, in general, aDVT was treated with catheter-directed thrombolysis (with or without thrombectomy), followed by percutaneous transluminal angioplasty and stent placement, with the WALLSTENT used in 78% of included studies.22 In another review, Husmann et al. included 11 patients treated with thrombolysis/thrombectomy followed by iliac venous WALLSTENT placement.23 These are examples of the literature supporting the efficacy of Boston Scientific’s WALLSTENT in treating patients after aDVT dissolution or extraction.
Venovo
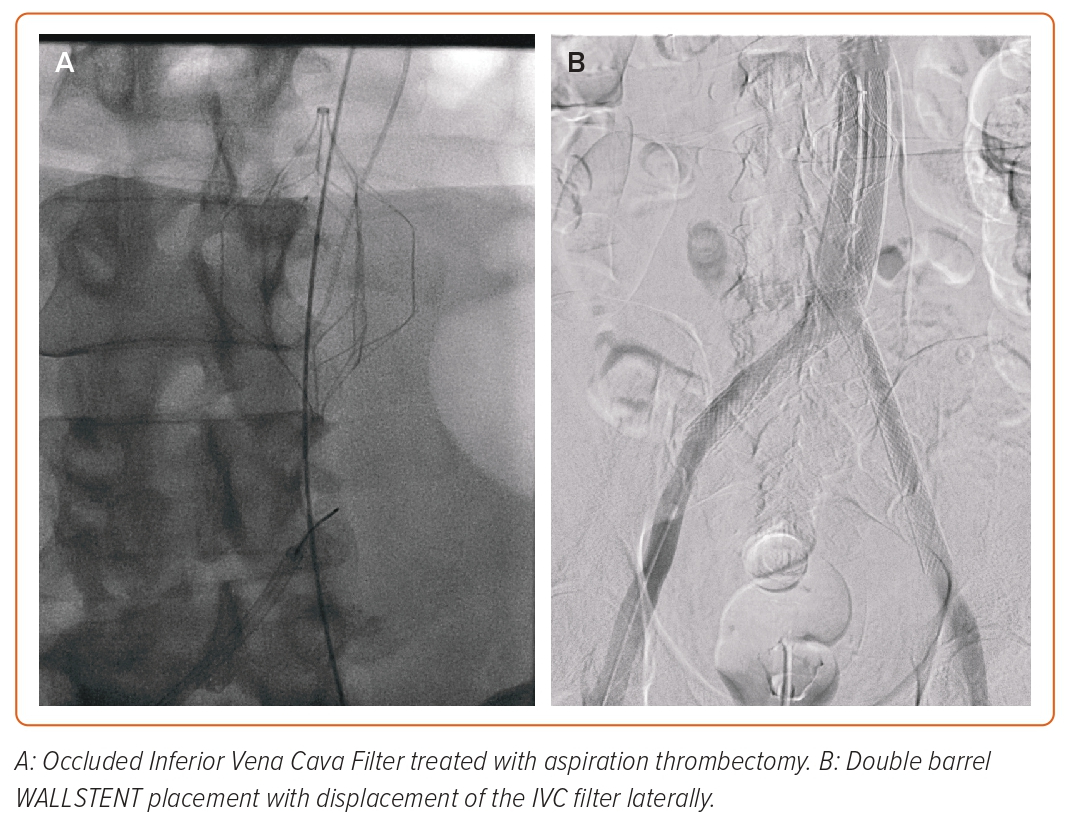
BD’s Venovo is an open-cell, self-expanding nitinol stent that comes in various sizes. The Venovo was the first stent with a venous indication approved by the Food and Drug Administration (in March 2019). The VERNACULAR Trial is a prospective single-arm multicentre worldwide study that has included 200 subjects with symptomatic iliofemoral venous outflow obstruction from sites throughout the US and Europe.24 The Venovo stent met its primary endpoint with excellent 36-month patency. The placement of Venovo stents following an iliofemoral venous thrombectomy is shown in Figure 2.
Figure 2: Venovo Placement Following Thrombectomy of Acute Iliofemoral Deep Venous Thrombosis
The 3-year results of the VERNACULAR trial were recently presented by Dake et al.24 The Venovo venous stent was successfully deployed in obstructive iliofemoral vein lesions and met the pre-specified primary outcome measures through 12 months. At 3 years, primary patency was 84%, reintervention rates were low, standardised quality of life and pain measures improved from baseline and there was no stent migration or fractures.24 However, the VERNACULAR trial did not enrol patients with aDVT.24
Abre
Medtronic’s Abre is an open-cell nitinol stent that comes in various sizes. The ABRE Study was a prospective interventional single-arm multicentre worldwide study that included 200 subjects with symptomatic iliofemoral venous outflow obstruction from 24 sites throughout the US and Europe.25 The study enrolled subjects across the spectrum of deep venous disease, including those with PTS (47%) and NIVL (36%), as well as those who presented with an aDVT (17%). The study also met its 12-month primary effectiveness endpoint, with an overall primary patency rate of 88.0% (162/184). Furthermore, the data demonstrated a freedom from clinically driven target lesion revascularisation rate of 92.4% (170/184) through 390 days.
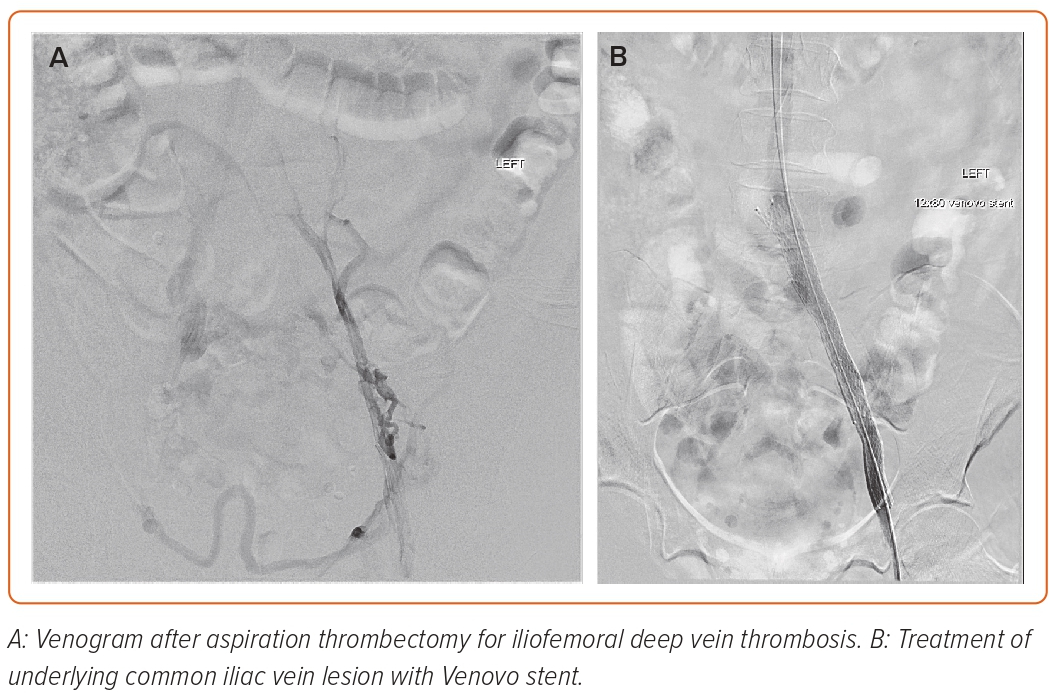
Notable secondary endpoint results from the ABRE Study included no stent fractures and no delayed stent migration observed within 12 months. In addition, there were sustained and statistically significant improvements in quality of life measures and venous functional assessment scores at 24 months compared with baseline. When the data are examined by the clinical presentation, they are compelling. The 2-year data were recently presented. At 24 months, the patency for stenting was 76.8% in the chronic DVT group, compared with 83.3% in the aDVT group.25 These positive data signal a benefit to treating patients with the Abre dedicated venous stent following thrombus dissolution/extraction when IVUS confirms an iliofemoral lesion. Successful Abre stent placement following thrombus removal is shown in Figure 3.
Figure 3: Abre Placement Following Thrombectomy of Acute Iliofemoral Deep Venous Thrombosis
Zilver Vena
The Zilver Vena is an open-cell, self-expanding nitinol stent that obtained a CE Mark in 2010. Cook Medical has completed the VIVO clinical trial evaluating the effectiveness of the Zilver Vena for iliofemoral occlusive disease.19,21 VIVO was a prospective single-arm multicentre worldwide study that included 243 subjects with symptomatic iliofemoral venous outflow obstruction from 24 sites throughout the US and Europe. The study included a real-world population, encompassing subjects across the spectrum of deep venous disease, including those with PTS (43%) and NIVL (33%), as well as those who presented with an aDVT (24%).19 Results though 2 years continue to support the safety and effectiveness of the Zilver Vena venous stent for the treatment of symptomatic iliofemoral venous outflow obstruction. In addition, the study revealed high rates of freedom from clinically driven reintervention, high rates of patency by ultrasound, clinical improvement (as measured by the VCSS) and no stent fractures.
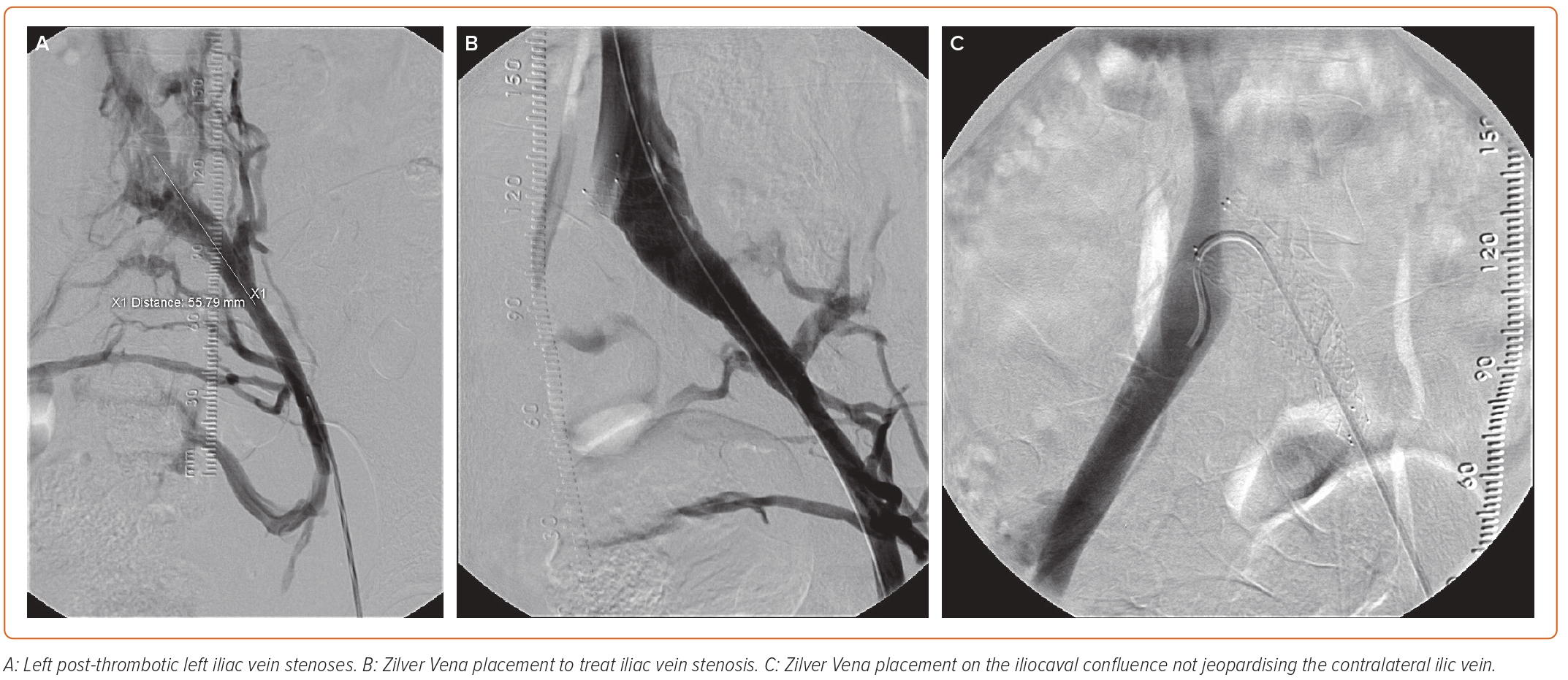
The Zilver Vena was demonstrated to have high patency by ultrasound at 2 years. In the aDVT groups, patency was 84% at 2 years, whereas in the chronic DVT (PTS) group patency was 86.1% at 2 years.26 Freedom from major adverse events was similar between the groups at 94.9% and 95.1% in the aDVT and chronic DVT (PTS) groups, respectively.26 There was a significant improvement in the mean VCSS from baseline (p<0.0001) in the VIVO study cohort at 1 month, and this improvement was maintained through 2 years. The mean VCSS decreased by 4.2 points from before the procedure through to 2 years. Subgroup analysis demonstrated the same trend for clinical improvement in each subgroup.19 These positive data signal a benefit of treating patients with the Zilver Vena dedicated venous stent following thrombus dissolution/extraction. Successful placement of a Zilver Vena on the iliocaval confluence after iliofemoral DVT thrombolysis is shown in Figure 4.
Figure 4: Zilver Vena Placement for Post-thrombotic Syndrome Stenosis
sinus-Venous and sinus-Obliquus
Optimed’s sinus-Obliquus is a purpose-built venous stent featuring a unique bevel for placement at the origin of the vena cava, a high-strength closed-cell segment for the iliac compression and a flexible segment for the external iliac. The sinus-Venous is a highly flexible, open-cell venous stent with independent power rings providing strength. The sinus-Venous device may be deployed in the iliac and femoral veins or as an extension stent to the sinus-Obliquus. TOPOS is a prospective multicentre single-arm study that included patients with PTS and iliofemoral venous outflow obstruction.27 The 2-year results from the TOPOS trial of Optimed’s sinus-Obliquus device for the treatment of PTS were recently presented at LINC 2022. At 24 months, TOPOS had a primary stent patency rate of 81% and a secondary patency rate of 96%.27 Patients in the study had a mean stent length of 22 cm, with 70% of stents extending beyond the inguinal ligament.27 Data at this time have not been reported on patients with acute DVT following thrombolysis/thrombectomy.
blueflow Venous Stent
A novel braided nitinol stent (blueflow Venous Stent; plus medica) provides additional flexibility due to its braided structure and high radial force due to its closed cell design. The BLUEFLOW Registry evaluated the mid-term effectiveness and safety of iliofemoral venous recanalisation using a novel braided venous stent in participants with chronic venous disease.28 The study demonstrated favourable primary patency at 12 months without major complications. Symptom severity, as measured by the revised VCSS (rVCSS) and clinical CEAP score, improved significantly, with an advantage for obstructions of the external over the common iliac vein. No significant difference in effectiveness could be found between thrombotic and non-thrombotic pathogenesis. The results suggest that deep venous stenting with the blueflow Venous Stent provides favourable primary patency and is associated with significant sustained improvement of symptom severity in a real-world population with obstructive chronic venous disease up to 12 months. This adds to the number of dedicated venous stents available for chronic DVT patients. Additional research is needed for aDVT patients.28
Z-stent
Stenting of the proximal iliocaval confluence deserves special consideration. Incomplete stent coverage in this area is a common cause of residual symptoms. IVUS coupled with bony landmarks as fluoroscopic markers is mandatory for accurate treatment. Extension of the iliac stent for a few centimetres into the IVC is generally required to traverse the proximal lesion in its entirety when using Boston Scientific’s WALLSTENT. An 18- or 20-mm stent dilated with 16- and 18-mm balloons, respectively, will accommodate most adults and provide a 2 mm reserve for extra dilation later, if required. Alternatively, a Z-stent (Cook) may be used proximally (within the WALLSTENT) for added radial strength under the artery and to minimise jailing of the contralateral iliac outflow.29 A significant reduction in contralateral DVT has been observed with the Z-stent extension compared with the WALLSTENT extension. The Z-stent has safety and efficacy in PTS patients, but additional research is needed for aDVT patients.
Single-institution Data on Venous Stenting for Patients With Acute Deep Venous Thrombosis and Post-thrombotic Syndrome
A few non-industry studies have examined differences in patency in patients with aDVT following thrombus removal and PTS stenoses. The recently published paper Venous Stent Patency is Independent of Total Stented Length in Non-thrombotic Iliac Vein and Post-thrombotic Venous Stenoses by Robertson et al. reported on 108 patients who underwent symptomatic iliofemoral venous stenting.30 All patients evaluated (58.3% female) had 6-month duplex ultrasound scans available for review. The mean (±SD) age of patients was 55.6±17.2 years. Overall, the 6-month patency was 89.9%. In the cohort, 56 (51.9%) patients had a total stented length ≤100 mm, with a 6-month patency of 92.9%, and 52 (48.1%) patients had a total stented length >100 mm, with a 6-month patency of 86.5%. There was no significant difference in the patency rate between the two groups (p=0.222).30 For patients with NIVL, the stent patency at 6 months was 98% (40/41). Patency for patients with PTS was determined to be 84% (32/38), whereas that for patients with aDVT who underwent stenting following thrombectomy was 86% (25/29).30 The total stented length was not predictive of loss of patency. In that study, the majority of stents used were Wallstent, Venovo and Abre, which supports their use in patients with aDVT and PTS.
In another paper, published by Tran et al. from the Heart and Vascular Institute, University of Pittsburgh Medical Center (Pittsburgh, PA, US), in 150 patients undergoing iliofemoral stenting for deep venous disease, adjunct IVUS assessment with multiplanar venography was associated with higher 30-day (98.6% versus 89.4%; p=0.02) and 2-year (90.3% versus 78.7%; p=0.03) primary patency than venography alone.26 In this study, 55.3% acute, 1.3% chronic or post-thrombotic and 23.3% of patients underwent intervention for NIVLs, with IVUS and venography-guided stenting compared between the groups. The patency of the stent group with aDVT was 88% compared with 86% in the post-thrombotic stenosis group, which was not statistically different.26 These data signal a benefit to stenting at the time of aDVT thrombectomy or thrombolysis. Tran et al. used a mixture of stents (61% and 47% Wallstent in the aDVT and chronic DVT patients, respectively). Importantly, the type of venous disease (i.e. aDVT versus chronic DVT or thrombotic versus non-thrombotic) was not found to be a risk factor for 30-day or 2-year stent failure in univariate or multivariate regression analyses.26 Lichtenberg et al. published data from the Arnsberg Aspirex Registry, including 56 patients with iliofemoral DVT.31 Patients were examined for acute and subacute DVTs, with 40 (71%) being acute. All patients underwent placement with dedicated venous stents following treatment with Aspirex. The cumulative patency after 1, 5 and 12 months was 95%, 94% and 87%, respectively.31 Clinical variables were also collected assessing for PTS. PTS analysis after 12 months was reported for 53 patients.31 Of these 53 patients, 34 (64%) were classified as low PTS (CEAP score <3, rVCSS <3), whereas 19 (36%) had moderate PTS (CEAP score >3, rVCSS >3).31 These compelling data support endovascular stent placement in aDVT patients following thrombus dissolution/extraction, as well as in PTS patients.
Conclusion
Raju and Neglén pioneered venous stenting, demonstrating its safety and efficacy for symptomatic iliofemoral outflow obstruction. Boston Scientific’s Wallstent has been the most frequently studied device for venous stenting in NIVL, aDVT and PTS patients. Data for patients with aDVT are accumulating for dedicated venous stents. Today, there is promising data accumulating from industry trials and non-industry-sponsored research. Medtronic’s and Cook’s industry-sponsored trials show the benefits of Abre and Zilver Vena, respectively, for stenting in patients with aDVT. Independent research has also revealed positive long-term results with venous stenting for aDVT patients following thrombus removal. More research is needed to identify the best practice for patients with aDVT and the best comprehensive approach. Currently, data from industry trials and from institutions across the world signal the benefit of venous stenting for patients with aDVT following thrombus removal. Although more data are needed, currently the results appear similar whether a venous stent is placed following thrombus removal for aDVT or for PTS.
- Chopard R, Albertsen IE, Piazza G. Diagnosis and treatment of lower extremity venous thromboembolism: a review. JAMA 2020;324:1765–76.
Crossref | PubMed - Kakkos SK, Gohel M, Baekgaard N, et al. Editor’s choice – European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg 2021;61:9–82.
Crossref | PubMed - Mazzolai L, Aboyans V, Ageno W, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J 2018;39:4208–18.
Crossref | PubMed - Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788–830.
Crossref | PubMed - Vedantham S, Grassi CJ, Ferral H, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol 2006;17:417–34.
Crossref | PubMed - Williams ZF, Dillavou ED. A systematic review of venous stents for iliac and venacaval occlusive disease. J Vasc Surg Venous Lymphat Disord 2020;8:145–53.
Crossref | PubMed - Raju S, Darcey R, Neglén P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg 2010;51:401–8.
Crossref | PubMed - Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007;46:979–90.
Crossref | PubMed - Raju S, Owen S, Neglen P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg 2002;35:8–15.
Crossref | PubMed - Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg 2002;35:694–700.
Crossref | PubMed - Cockett FB, Thomas ML, Negus D. Iliac vein compression.–Its relation to iliofemoral thrombosis and the post-thrombotic syndrome. Br Med J 1967;2:14–9.
Crossref | PubMed - Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord 2017;5:678–87.
Crossref | PubMed - Deatrick KB, Elfline M, Baker N, et al. Postthrombotic vein wall remodeling: preliminary observations. J Vasc Surg 2011;53:139–46.
Crossref | PubMed - Marston W. Anatomy, technique, and anticoagulation are the main drivers of success. Presented at: 34th Annual American Venous Forum 2022, Orlando, FL, 23 February 2022.
- Rossi FH, Kambara AM, Izukawa NM, et al. Randomized double-blinded study comparing medical treatment versus iliac vein stenting in chronic venous disease. J Vasc Surg Venous Lymphat Disord 2018;6:183–91.
Crossref | PubMed - O’Donnell TF, Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society of Vascular Surgery and the American Venous Forum. J Vasc Surg 2014;60(2 Suppl):3S–59S.
Crossref | PubMed - Wittens C, Davies AH, Bækgaard N, et al. Editor’s choice – management of chronic venous disease: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2015;49:678–737.
Crossref | PubMed - Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord 2018;6:48–56.e1.
Crossref | PubMed - O’Sullivan GJ, Karunanithy N, Binkert CA, et al. One year outcomes of the VIVO-EU study of treatment of symptomatic iliofemoral outflow obstruction with the Zilver Vena venous self-expanding stent. Cardiovasc Intervent Radiol 2021;44:1930–6.
Crossref | PubMed - Raju S, Ward M jr, Kirk O. A modification of iliac vein stent technique. Ann Vasc Surg 2014;28:1485–92.
Crossref | PubMed - Murphy EH, Johns B, Varney E, et al. Deep venous thrombosis associated with caval extension of iliac stents. J Vasc Surg Venous Lymphat Disord 2017;5:8–17.
Crossref | PubMed - Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv 2015;8:e002772.
Crossref | PubMed - Husmann MJ, Heller G, Kalka C, et al. Stenting of common iliac vein obstructions combined with regional thrombolysis and thrombectomy in acute deep vein thrombosis. Eur J Vasc Endovasc Surg 2007;34:87–91.
Crossref | PubMed - Dake MD, O’Sullivan G, Shammas NW, et al. Three-year results from the Venovo venous stent study for the treatment of iliac and femoral vein obstruction. Cardiovasc Interv Radiol 2021;44:1918–29.
Crossref | PubMed - Murphy E. ABRE study 24 month outcomes and subgroup analyses. Presented at: LINC 2022, Leipzig, Germany, 6 June 2022.
- Tran LM, Go C, Zaghloul M. Adjunct intravascular ultrasound (IVUS) is associated with improved midterm stent patency in deep venous interventions. Presented at: 33rd Annual American Venous Forum 2021, Virtual, 17–20 March 2021.
- Sebastian T. The TOPOS study: 2-year clinical outcomes of a hybrid oblique nitinol stent for patients with postthrombotic syndrome and common iliac vein compression. Presented at: LINC 2022, Leipzig, Germany, 7 June 2022.
- Lichtenberg M, Stahlhoff S, Özkapi A, de Graaf R. Braided nitinol stent for chronic iliofemoral venous disease – the real-world BLUEFLOW registry. Vasa 2021;50:372–7.
Crossref | PubMed - Jayaraj A, Noel C, Raju S. Contralateral limb improvement after unilateral iliac vein stenting argues against simultaneous bilateral stenting. J Vasc Surg Venous Lymphat Disord 2020;8:565–71.
Crossref | PubMed - Robertson B, Shapiro J, Muck A, et al. Venous stent patency is independent of total stented length in non-thrombotic iliac vein and post-thrombotic venous stenoses. J Vasc Surg Venous Lymphat Disord 2022.
epub ahead of press.
Crossref | PubMed - Lichtenberg M, Stahlhoff WF, Özkapi A, et al. Safety, procedural success and outcome of the Aspirex®S endovascular thrombectomy system in the treatment of iliofemoral deep vein thrombosis – data from the Arnsberg Aspirex registry. Vasa 2019;48:341–6.
Crossref | PubMed