Maria Joh,Kush R Desai
|
Abstract Non-thrombotic iliac vein lesions (NIVLs) refer to iliac vein lumen stenosis, usually secondary to extrinsic compression, without associated thrombosis. Clinical presentation varies; patients may be asymptomatic, have symptoms of lower extremity venous hypertension, or in women, may be associated with chronic pelvic pain. Given the significant variability in symptomatology, thorough history and physical examination are mandatory in excluding other causes of symptoms. Non-invasive imaging, such as venous duplex/insufficiency ultrasound examinations, and axial imaging aid in the diagnosis of a NIVL in the appropriate clinical context. Catheter venography and intravascular ultrasound remain the primary modalities for definitive diagnosis, treatment planning, and ultimately placement of self-expanding venous stents to resolve the causative iliofemoral venous obstruction. In appropriately selected patients, stent placement can lead to marked improvements in symptoms, heal stasis ulceration when present, and improve disease-specific and overall quality of life. Stents placed in patients with NIVL demonstrate high long-term primary patency. In this article, the authors discuss clinical presentation, diagnostic workup, endovascular interventions and outcomes of NIVL treatment. Keywords: Iliac vein compression, May–Thurner syndrome, chronic venous insufficiency, venous stents Disclosure: KRD reports speaker’s bureau/consulting for Cook Medical, Boston Scientific, Becton Dickinson/Bard, Medtronic, Penumbra, Tactile Medical; consultant for Philips, W.L. Gore, Tactile Medical, Shockwave Medical, Asahi Intecc; and is a Deputy Editor of Vascular & Endovascular Review, this did not influence peer review. MJ has no conflicts of interest to declare. Received: 20 August Accepted: 11 January 2022Published online: 09 June 2022 Citation: Vascular & Endovascular Review 2022;5:e05. DOI:https://doi.org/10.15420/ver.2021.11 Correspondence Details: Kush R Desai, Department of Radiology, Northwestern University, 676 N St Clair St, Suite 800, Chicago, IL 60611, US. E: kdesai007@northwestern.edu
This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly
|
In 1908, McMurrich studied 107 cadavers and found 35 had adhesions in the iliac veins, the majority of which were on the left side.1 Subsequently in 1943, Ehrich and Krumbhaar found that 95 of 412 cadavers had obstructive lesions consisting of collagen and elastin in the left iliac veins.2 This was further characterised by May and Thurner in 1957 as ‘spurs’ in the left iliac veins that may predispose to venous thrombosis because of disruption of blood flow.3 This condition was referred to as ‘iliac vein compression’ by Cockett and Thomas in 1965.4,5 These initial studies did not attempt to differentiate between non-thrombotic iliac vein lesions (NIVLs) and thrombotic lesions. However, more recent evidence suggests that clinical outcome and recurrence rates may differ, necessitating distinction between the two lesion types.
NIVLs refer to iliac vein lumen stenosis, usually secondary to extrinsic compression, without associated thrombosis. Most commonly NIVLs are caused between arterial structures and the spine, and are associated with intrinsic vein stenosis characterised by wall fibrosis or intraluminal webs/spurs. When there is compression of the left common iliac vein by the right common iliac artery, it is commonly referred to as May–Thurner syndrome or Cockett’s syndrome.6
Patients with NIVLs may develop symptoms of chronic venous insufficiency (CVI), although the true prevalence of NIVL-associated CVI is unknown. Symptoms of CVI can significantly decrease quality of life and increase healthcare costs.7,8 In patients with significant persistent symptoms despite conservative management, endovascular stent placement has been used as a safe and effective treatment option for NIVLs.9 To achieve treatment success, patient selection is crucial to appropriately exclude patients experiencing symptoms because of other aetiologies.
In this article, we will discuss the clinical presentation, diagnostic workup and endovascular interventions of NIVLs, along with the outcomes of treatment.
Clinical Presentation
Clinical presentation of NIVL varies, ranging from asymptomatic patients to those with symptoms of CVI. Several studies have found that a significant portion of patients with NIVL may not have appreciable symptoms.10–12 One study retrospectively reviewed 50 abdominal/pelvic CT scans of patients presenting with abdominal pain to the emergency department; there were no lower extremity symptoms or prior deep venous thrombosis (DVT).11 Among these subjects, 33 (66%) had >25% diameter compression, which corresponds to approximately 50% area stenosis.11 In a more recent study, angiography of the iliac veins was performed prospectively in 20 healthy volunteers, among whom 19 had at least two angiographic findings consistent with significant iliac vein obstruction (including contrast translucency, lumen deformity, as well as axial, transpelvic or ascending lumbar collaterals). Sixteen of these subjects had no visible or palpable signs of venous disease (C0 by Clinical Etiology Anatomy Pathophysiology [CEAP] classification) and four of them were minimally symptomatic with telangiectasias/reticular veins (C1 by CEAP classification).12 Thus, the presence of a NIVL by imaging does not directly correlate to the presence of clinically symptomatic venous obstructive disease.
Proposed Algorithm for Diagnosis and Management of Non-thrombotic Iliac Vein Lesions
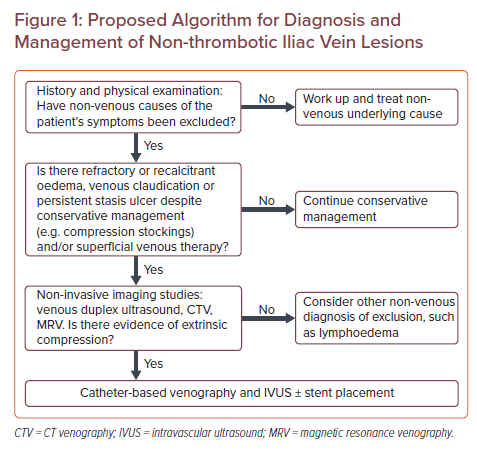
Symptomatic patients with NIVLs experience varying degrees of CVI symptoms in the lower extremities. These symptoms include – but are not limited to – lower extremity heaviness, discomfort, pain, oedema, varicose veins, telangiectasia or, in its most severe form, ulcers, in the affected lower extremity. Women with NIVLs may experience additional symptoms including chronic pelvic pain, dyspareunia, vulvar swelling/pressure, and superficial lower extremity pelvic-derived varicosities.
Given the wide range of presentation of NIVL, it has been hypothesised that NIVL is a permissive lesion, and factors such as vessel trauma or oedema are likely to precipitate further symptom manifestation.13 However, it remains unclear in which instances these lesions become clinically significant; a clinical conundrum, given a significant portion of the population has clinically silent compression that does not need treatment. Therefore, screening of asymptomatic patients currently remains impractical. Rather, these studies highlight the importance of appropriately selecting patients with symptoms directly attributable to iliac vein obstruction to maximise the potential benefits of endovascular treatment.
Approach to Diagnostic Workup
Diagnostic workup for a patient suspected to have a NIVL should begin with thorough history and physical examination to look for other aetiologies of the patient’s symptoms, including but not limited to medications (e.g. calcium channel blockers), congestive heart failure, lymphoedema, liver disease and endocrine dysfunction. Once other nonvascular aetiologies have been excluded and NIVL is suspected to be a major contributor to the patient’s symptoms, further evaluation for diagnosis and characterisation of NIVL may be pursued (Figure 1). Given that CVI more frequently affects women, female patients should routinely and specifically be assessed for chronic pelvic pain symptoms.14,15
The severity of symptoms can be scored using the revised Venous Clinical Severity Score (VCSS). This can be supplemented by the CEAP system, although this assessment lacks precision and simply reports the most severe physical manifestation of venous disease, not taking into account functional limitaitons.16–18 At this time, there is no disease severity scoring metric that is specific to NIVL. Regardless of the classification system used, a baseline disease severity should be documented to allow assessment of clinical progression or improvement after treatment.
Imaging studies aid in diagnosis and treatment planning. Initial non-invasive workup should include a venous duplex ultrasound, which may directly visualise compression or obstruction of the iliac vein as well as exclude venous thrombosis and post-thrombotic changes. Venous insufficiency or reflux should be assessed with augmentation techniques. Ultrasound evaluation of the iliac vein may be limited by technical factors (including operator experience, body habitus, or overlying bowel gas) and may fail to directly visualise the pathology within the iliac vein. In these cases, indirect findings of a clinically significant iliac vein lesion may be utilised. These include pre-stenotic vein dilatation, absent respiratory phasicity, internal iliac vein flow reversal and poor response to augmentation.19–23 In addition to duplex ultrasound, axial imaging may aid in non-invasive diagnosis. CT or magnetic resonance venography (MRV) can identify extrinsic compression, as well as concurrent thrombosis or presence of collateral veins. Limitations of cross-sectional imaging includes variable sensitivity depending on the hydration status of the patient, ionizing radiation with the use of CT, and necessity for local expertise with MRV.24 Catheter-based venography and intravascular ultrasound (IVUS) are the primary interventional imaging modalities for diagnosis and treatment of NIVLs. With IVUS, high resolution circumferential sonographic imaging with high frequency probes leads to improved accuracy of diagnosing venous lesions, such as webs or spurs.24–28 Additionally, IVUS is critical to improved accuracy of luminal measurements for treatment planning and stent size selection, as well as assessment following stent placement.29,30
Endovascular Management
Asymptomatic patients with NIVLs discovered incidentally are not candidates for endovascular intervention. In these patients, counselling on risks of developing symptoms of CVI including DVT may be indicated, though has not been prospectively validated.31–34 Conservative management should always be pursued first in patients with symptoms of CVI, including regular use of compression stockings. Endovascular management should be considered in patients with moderate to severe symptoms and those who are refractory to conservative management. For patients with superficial venous pathology that is thought to be a significant contributing cause to their symptoms, superficial treatment should be considered prior to iliac vein stent placement.
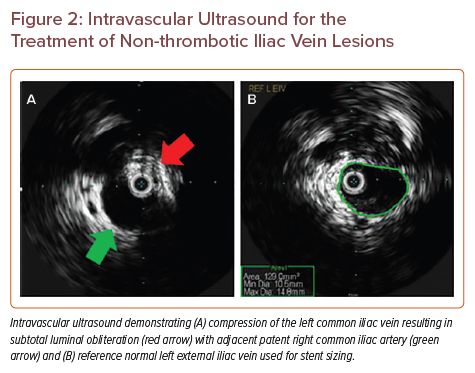
The primary objective of intervention for an obstructive venous lesion is to establish adequate inflow and outflow. Studies have found that angioplasty without stent placement for NIVL is generally not sufficient because of lesion recoil, therefore stent placement is the primary endovascular treatment modality for NIVL.35 The interventional procedure involves a series of general technical steps. First, ultrasound-guided venous access is obtained below the level of disease, typically in the ipsilateral common femoral, cranial great saphenous, or femoral vein with placement of a vascular sheath. Initial venography is performed to assess the lesion and the degree of compression/obstruction, presence of pre-stenotic dilation and venous collaterals. IVUS is then used to further assess the obstructive venous lesion and obtain measurements to determine the size of the stent. The size of the stent is chosen based on the size of the adjacent normal segment (Figure 2). In our practice, we use a normal ipsilateral external iliac vein; care must be taken to not measure pre-stenotic dilation as a reference segment as this can lead to incorrect stent sizing. A self-expanding stent is then deployed, extending into the external iliac vein, generally with a goal to extend the stent around the natural curve of the external iliac vein such that adequate stent anchoring in normal anatomy occurs, to minimise the risk of stent migration. Wallstent (Boston Scientific) is a type of elgiloy stent, which has now been approved for treatment of iliofemoral venous obstruction. Additionally, self-expanding nitinol stents such as VICI (Boston Scientific), Venovo (Becton Dickinson/Bard), Abre (Medtronic), and Zilver Vena (Cook Medical) have also been approved for this application in the United States. In Europe, Sinus Venous (Optimed) and BlueFlow (Plus Medica) are additional options.36
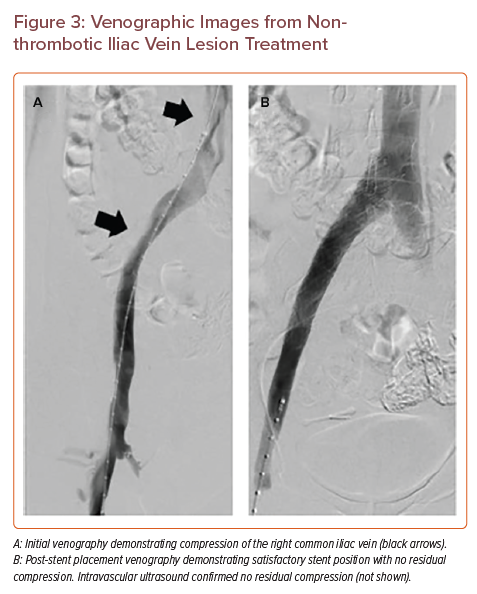
Historically, lesions with >50% area stenosis (corresponding to approximately 30% diameter stenosis) were considered candidates for stent placement.6,35,37 More recent evidence suggests otherwise. In the prospective, single-arm VIDIO trial, 68 patients were included with CEAP C4–6 who had a stent placement, 48 of whom had NIVL (20 had post-thrombotic lesions).30 The study found no correlation between area of stenosis and outcome, and an area of receiver operating characteristic curve analysis demonstrated clinical improvement when a higher threshold of >61% diameter stenosis was used in NIVL.30 Based on this prospective trial, we suggest consideration of stent placement in patients with lesions that have >61% diameter stenosis relative to an average reference diameter in a normal venous segment (in our practice, this is frequently the external iliac vein). After placement of a stent, repeat venography and IVUS confirm appropriate stent position/apposition and the presence of unimpeded flow (Figure 3). Collaterals, if initially present, can be reassessed for resolution at this step, suggestive of a therapeutic outcome. Currently, there is no consensus on the use of anticoagulation or anti-platelet agents after stent placement.38 We do not routinely prescribe anticoagulation after NIVL stent placement.
In women with NIVL and coexistent ovarian vein reflux with symptoms of chronic pelvic pain, treatment should be directed according to the dominant symptomatic feature. For such patients who primarily present with pelvic pain and minimal lower extremity symptoms, we suggest ovarian vein embolisation prior to stent placement for NIVL. Patients who have a dominant feature of lower extremity symptoms, including venous claudication, we suggest consideration of initial stent placement. The patient should be reassessed at follow-up, with staged stent placement for NIVL or ovarian vein embolisation if symptoms are refractory or only minimally improved.
Outcomes and Complications
Stent placement has been shown to improve pain and swelling, ulcer healing and quality of life in NIVL patients.9,39,40 Oedema relief was reported in nearly 90% of the patients.41 Ulcer healing was reported in more than 80% patients, with one study reporting even better healing in patients with NIVL compared to post-thrombotic iliac vein lesions with 5-year healing rates of 87% and 66%, respectively.41,42
Multiple studies have reported excellent patency rates for endovascular treatment of NIVLs.9,43,44 A meta-analysis reported primary patency of 94.1% at 1 year and 88.9% at 3 years. Assisted primary-secondary patency rates were 100% at 3 years.45 In the recent prospective, multicentre trial VIRTUS, self-expanding nitinol stents were placed successfully in 170 symptomatic patients with >50% stenosis. At 1 year, the primary patency rate was 98.4% in the NIVL limb, compared with 79.8% in the post-thrombotic limb.36 In this study, which included patients with VCSS of ≥2 or CEAP ≥C3, 64% of patients (including both NIVL and post-thrombotic limbs) demonstrated at least a 3-point reduction in VCSS in 1 year, with 5-point average reduction specifically in the NIVL limb.36 The majority (58%) of the patients (including both NIVL and post-thrombotic limbs) reported improvement in quality of life in 1 year with at least a 9-point reduction in the Chronic Venous Insufficiency Quality of Life Questionnaire-20, with 14-point average reduction specifically in the NIVL limb.36
Intravascular Ultrasound for the Treatment of Non-thrombotic Iliac Vein Lesions
Venographic Images from Nonthrombotic Iliac Vein Lesion Treatment
The rate of development of in-stent restenotic material is lower in NIVL treatment compared with post-thrombotic lesion treatment. One study reported cumulative rate of material accumulation resulting in >50% stenosis to occur approximately in 1% of stents placed for NIVL lesions, compared to 10% for post-thrombotic lesions.9
The most common complication after endovascular treatment of NIVL is access-site-related complications including bleeding, haematomas or arteriovenous fistulas; the risk of the latter is further reduced by use of ultrasound guidance.6,44 Another common complication includes, but is not limited to, back pain from stents, which is often self-limited.41 One specific complication of iliac vein stent placement is contralateral common iliac vein thrombosis from its exclusion in the case of stent extension into the inferior vena cava, though this is uncommon.46,47 Rare complications such as stent fracture, malposition, migration or erosion have also been reported.41
Future Directions
At this time, there is no consensus or guideline to suggest use of any specific stent for treatment of NIVL (for instance, those designed specifically for treatment of May–Thurner syndrome versus standard stents), although this would warrant further investigation. Additional areas for investigation include refinement of our understanding of what constellation of symptoms constitute a clinically relevant NIVL, identification of validated diagnostic criteria for NIVL given the presence of specific symptoms and procedural endpoints and outcomes measurements to demonstrate clinical improvement following treatment.
Conclusion
Appropriate initial patient selection is key to a successful outcome in the endovascular treatment of NIVL. Further studies are needed to determine which lesional or clinical features lead to a NIVL becoming clinically symptomatic, given that a significant number of NIVLs are clinically silent and do not merit treatment. Studies have demonstrated that stent placement is safe and effective with largely positive outcomes when performed at experienced centres in patients where it is clinically indicated.
-
- McMurrich JP. The occurrence of congenital adhesions in the common iliac veins, and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci 1908;135:342–6.
Crossref - Ehrich WE, Krumbhaar EB. A requent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J 1943;26:18–31.
Crossref - May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957;8:419–27.
Crossref| PubMed - Cockett FB, Thomas ML, Negus D. Iliac vein compression –its relation to iliofemoral thrombosis and the post-thrombotic syndrome. Br Med J 1967;2:14–9.
Crossref| PubMed - Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg 1965;52:816–21.
Crossref| PubMed - Mahnken AH, Thomson K, de Haan M, O’Sullivan GJ. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol 2014;37:889–897.
Crossref| PubMed - Chiesa R, Marone EM, Limoni C, et al. Effect of chronic venous insufficiency on activities of daily living and quality of life: correlation of demographic factors with duplex ultrasonography findings. Angiology 2007;58:440–9.
Crossref| PubMed - Tsai S, Dubovoy A, Wainess R, et al. Severe chronic venous insufficiency: magnitude of the problem and consequences. Ann Vasc Surg 2005;19:705–11.
Crossref| PubMed - Neglen P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007;46:979–90.
Crossref| PubMed - Oguzkurt L, Ozkan U, Ulusan S, et al. Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol 2008;19:366–70.
Crossref| PubMed - Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an asymptomatic patient population. J Vasc Surg 2004;39:937–43.
Crossref| PubMed - van Vuuren T, Kurstjens RLM, Wittens CHA, et al. Illusory angiographic signs of significant iliac vein compression in healthy volunteers. Eur J Vasc Endovasc Surg 2018;56:874–9.
Crossref| PubMed - Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg 2006;44:136–44.
Crossref| PubMed - Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol 2005;15:175–84.
Crossref| PubMed - Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation 2014;130:333–46.
Crossref| PubMed - Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord 2020;8:342–52.
Crossref| PubMed - Marston WA, Vasquez MA, Lurie F, et al. Multicenter assessment of the repeatability and reproducibility of the revised Venous Clinical Severity Score (rVCSS). J Vasc Surg Venous Lymphat Disord 2013;1:219–24.
Crossref| PubMed - Vasquez MA, Rabe E, McLafferty RB, et al. Revision of the venous clinical severity score: venous outcomes consensus statement. J Vasc Surg 2010;52:1387–96.
Crossref| PubMed - Hui JZ, Goldman RE, Mabud TS, et al. Diagnostic performance of lower extremity Doppler ultrasound in detecting iliocaval obstruction. J Vasc Surg Venous Lymphat Disord 2020;8:821–30.
Crossref| PubMed - Sloves J, Almeida JI. Venous duplex ultrasound protocol for iliocaval disease. J Vasc Surg Venous Lymphat Disord 2018;6:748–57.
Crossref| PubMed - Jain AK, Soult MC, Resnick SA, et al. Detecting iliac vein thrombosis with current protocols of lower extremity venous duplex ultrasound. J Vasc Surg Venous Lymphat Disord 2018;6:724–9.
Crossref| PubMed - Metzger PB, Rossi FH, Kambara AM, et al. Criteria for detecting significant chronic iliac venous obstructions with duplex ultrasound. J Vasc Surg Venous Lymphat Disord 2016;4:18–27.
Crossref| PubMed - Khilnani NM. Duplex ultrasound evaluation of patients with chronic venous disease of the lower extremities. AJR Am J Roentgenol 2014;202:633–42.
Crossref| PubMed - Toh MR, Tang TY, Lim H, et al. Review of imaging and endovascular intervention of iliocaval venous compression syndrome. World J Radiol 2020;12:18–28.
Crossref| PubMed - Forauer AR, Gemmete JJ, Dasika NL, et al. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol 2002;13:523–7.
Crossref| PubMed - Neglen P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg 2002;35:694–700.
Crossref| PubMed - Hurst DR, Forauer AR, Bloom JR, et al. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg 2001;34:106–13.
Crossref| PubMed - Raju S, Martin A, Davis M. The importance of IVUS assessment in venous thrombolytic regimens. J Vasc Surg Venous Lymphat Disord 2013;1:108.
Crossref| PubMed - Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord 2017;5:678–87.
Crossref| PubMed - Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord 2018;6:48–56.E1.
Crossref| PubMed - Chen F, Den J, Yuan QW, et al. Compression of left common iliac vein is independently associated with left-sided deep vein thrombosis. J Vasc Surg Venous Lymphat Disord 2013;1:364–9.
Crossref| PubMed - Birn J, Vedantham S. May-Thurner syndrome and other obstructive iliac vein lesions: meaning, myth, and mystery. Vasc Med 2015;20:74–83.
Crossref| PubMed - Chan KT, Tye GA, Popat RA, et al. Common iliac vein stenosis: a risk factor for oral contraceptive-induced deep vein thrombosis. Am J Obstet Gynecol 2011;205:537.e531–6.
Crossref| PubMed - Carr S, Chan K, Rosenberg J, et al. Correlation of the diameter of the left common iliac vein with the risk of lower-extremity deep venous thrombosis. J Vasc Interv Radiol 2012;23:1467–72.
Crossref| PubMed - Raju S. Treatment of iliac-caval outflow obstruction. Semin Vasc Surg 2015;28:47–53.
Crossref| PubMed - Razavi MK, Black S, Gagne P, et al. Pivotal study of endovenous stent placement for symptomatic iliofemoral venous obstruction. Circ Cardiovasc Interv 2019;12:e008268.
Crossref| PubMed - Raju S, Tackett P, Jr., Neglen P. Reinterventions for nonocclusive iliofemoral venous stent malfunctions. J Vasc Surg 2009;49:511–8.
Crossref| PubMed - Tran MA, Lakhanpal P, Lakhanpal S, et al. Type of anti-thrombotic therapy for venous stenting in patients with non-thrombotic iliac vein lesions does not influence the development of in-stent restenosis. Phlebology 2020;35:805–13.
Crossref| PubMed - Raju S, Darcey R, Neglen P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg 2010;51:401–88.
Crossref| PubMed - Liu Z, Gao N, Shen L, et al. Endovascular treatment for symptomatic iliac vein compression syndrome: a prospective consecutive series of 48 patients. Ann Vasc Surg 2014;28:695–704.
Crossref| PubMed - Ye K, Lu X, Li W, et al. Long-term outcomes of stent placement for symptomatic nonthrombotic iliac vein compression lesions in chronic venous disease. J Vasc Interv Radiol 2012;23:497–502.
Crossref| PubMed - Raju S, Kirk OK, Jones TL. Endovenous management of venous leg ulcers. J Vasc Surg Venous Lymphat Disord 2013;1:165–72.
Crossref| PubMed - Raju S, Owen S, Jr., Neglen P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg 2002;35:8–15.
Crossref| PubMed - Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv 2015;8:e002772.
Crossref| PubMed - Wen-da W, Yu Z, Yue-Xin C. Stenting for chronic obstructive venous disease: a current comprehensive meta-analysis and systematic review. Phlebology 2016;31:376–89.
Crossref| PubMed - Khairy SA, Neves RJ, Hartung O, O’Sullivan GJ. Factors associated with contralateral deep venous thrombosis after iliocaval venous stenting. Eur J Vasc Endovasc Surg 2017;54:745–51.
Crossref| PubMed - Gloviczki P, Lawrence PF. Iliac vein stenting and contralateral deep vein thrombosis. J Vasc Surg Venous Lymphat Disord 2017;5:5–6.
Crossref| PubMed
- McMurrich JP. The occurrence of congenital adhesions in the common iliac veins, and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci 1908;135:342–6.
