|
Abstract Renal injuries are the most common urinary tract injury secondary to external abdominal trauma. They are caused by blunt, penetrating and iatrogenic mechanisms. Despite the high number of blunt renal injuries, little evidence is available to guide management, especially with the evolution of embolisation as a minimally invasive treatment. Consequently, clinical practice is driven by results of observational studies and anecdote. We have reviewed the current trends in practice when using renal artery embolisation in the management of blunt renal injuries. Three key principles are highlighted. First, high-grade blunt renal injuries can be successfully managed with embolisation. Second, embolisation should be considered when there is radiological evidence of active contrast extravasation, pseudoaneurysm or arteriovenous fistula. Third, embolisation can be used to manage blunt renal injuries in haemodynamically unstable patients. Beyond this, evidence regarding optimal technique, CT indications, clinical status, comorbidities and complications are inconclusive. We discuss the implications for clinical practice and how these findings should define the agenda for future clinical research. Keywords Disclosure: RL is on the editorial board for Vascular and Endovascular Review; this did not influence peer review. All other authors have no conflicts of interest to declare. Received: Accepted: Published online: Correspondence Details: Rosemary Denning Ho, Hull York Medical School, University of York, University Rd, York YO10 5DD, UK. E: rdh@doctors.org.uk Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly. |
Renal injuries occur in approximately 1–5% of all patients with trauma and are the most common urinary tract injury secondary to external abdominal trauma.1 Renal trauma mainly arises from blunt, penetrating and iatrogenic mechanisms.2,3 Despite how common blunt renal injuries (BRIs) are, little evidence is available to guide management. Patients can undergo operative management or non-operative management and the use of expectant management, embolisation and surgery vary from institution to institution.
We aim to review the current trends concerning renal artery embolisation in the management of BRIs. We examine the evidence for indications for embolisation, outcomes of embolisation and its comparison to surgical and other non-operative adjunctive management, technical considerations, injury characteristics warranting embolisation and complications. Based on the evidence, we propose a treatment algorithm for the management of patients with BRI.
The Evidence
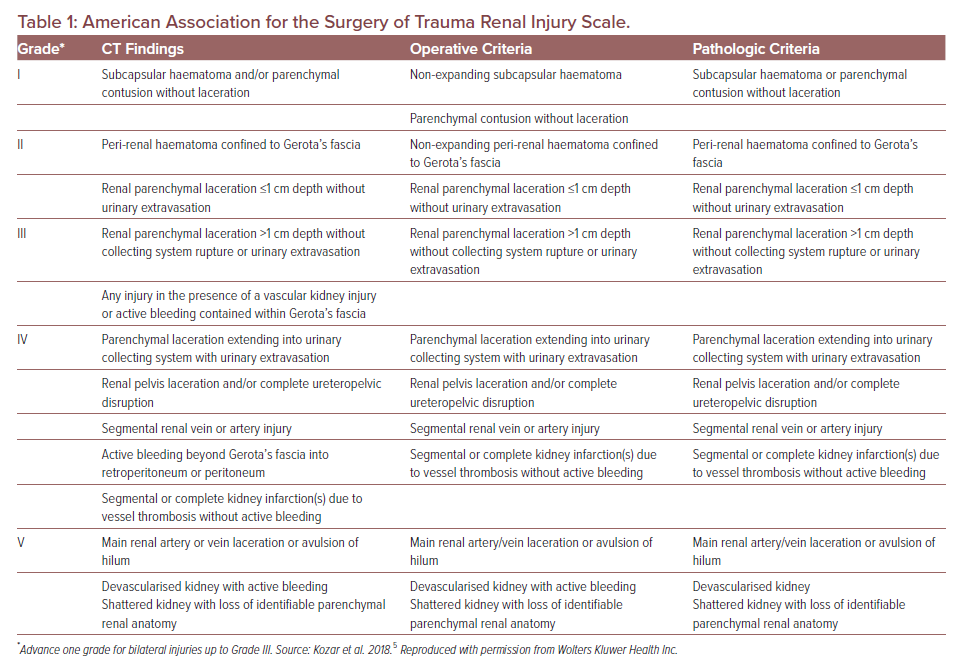
American Association for the Surgery of Trauma Grade
BRIs are usually identified using helical CT.4 Injuries are graded according to the American Association for the Surgery of Trauma (AAST) grading system. The AAST grading system was first described in 1989 and proposed to classify injury severity and guide management for a particular organ and was revised in 2018.5 The revised AAST grading for kidney injuries is shown in Table 1. Severity is classified according to the depth of parenchymal injury and involvement of ureteric or renal vasculature.
Most BRIs are low-grade (AAST <2) and non-life threatening.2,3 Renal artery embolisation (RAE) has been used to manage lower-grade BRIs (AAST ≤2), but since most of these injuries are minor and self-limiting, they can be managed conservatively as per the European Association of Urology (EAU) guidelines.6,7
For high-grade renal injuries (AAST ≥3), surgery has been traditionally preferred. There are no validated criteria to identify patients with BRIs requiring RAE by AAST grade. One study found that increasing AAST grade was an independent predictor for clinicians performing RAE in patients with BRIs (Grade 1: OR 0.4; Grade 2: OR 1.05; Grade 3: OR 1.49; Grade 4: OR 3.52; Grade 5: not reported).8 Other studies have grouped patients with both blunt and penetrating mechanisms of injury in their analyses, making it difficult to determine RAE success or failure as it is unclear whether blunt versus penetrating injuries could be confounders. These studies have shown that RAE is likely to be beneficial in patients with high-grade renal trauma (AAST >3).9–12
RAE in Grade V BRIs has been reported in the literature.6,9,13–23 However, the optimal strategy for Grade V kidney trauma remains uncertain as success rates have been variable and repeat embolisation may be necessary.6,9,15,17,22,24 More promising results show that when used in the management of Grade V parenchymal and renovascular lesions, RAE can result in minimal complications and retains an excellent likelihood of preserving the maximal amount of functional renal parenchyma without the need for further intervention, even despite haemodynamic instability.16–18,22 Vascular injury with shattered kidneys in the presence of no suspicions of a pedicle injury using CT could be an indication for RAE.17,25
Grade V renal pedicle avulsion is usually managed with surgery as it is technically demanding, but advances in angioembolisation have likely shifted practice patterns.26 Renal pedicle avulsions may now be possible with RAE due to vasospasm and the use of various sizes of coils to scaffold and anchor the bleeding vessel.14 This discrepancy in findings for the use of RAE in Grade V injury could pertain to differences in injury mechanisms, techniques used by interventional radiologists or the lack of uniformity of Grade V renal injuries. Differences in the sample sizes of studies may also account for the variable success rates. Increasing the sample size in future studies will help combat this uncertainty.
CT Criteria
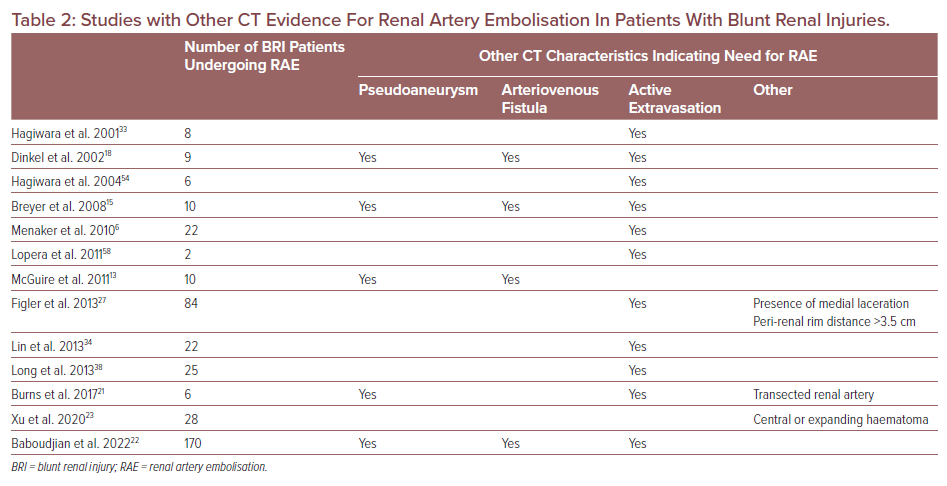
Retrospective studies have identified other findings from CTs in patients sustaining BRIs who were treated with RAE (Table 2). Active contrast extravasation, pseudoaneurysm and arteriovenous fistulae were the most reported indications. Of interest is a single-centre American study (n=84) showing that the presence of at least two high-risk criteria on CT (intravascular contrast extravasation, presence of medial laceration, peri-renal haematoma rim distance >3.5 cm) predicted the need for intervention, including angioembolisation, in the management of Grade IV BRIs with haemodynamic instability.27 The findings from this and other studies have been corroborated by a survey of clinicians regarding the management of BRI, which concluded that CT evidence of active arterial bleeding, pseudoaneurysm and/or arteriovenous fistula (AVF) and discontinuity of Gerota’s fascia may be associated with the need for RAE.28
Outcomes from Renal Artery Embolisation
Complete control of bleeding using embolisation for BRI was specified in eight studies after the first attempt, and in three studies after multiple attempts.11,16–19,21,29–32,33 Repeat embolisation was associated with inadequate haemostasis leading to persistent bleeding and higher renal injury grade.23–25
In some cases, there was an occasional need for nephrectomy and even exploratory laparotomy following failed embolisation, although these were in older studies.6,14,22,24,25,34–37 The most common indication for post-embolisation surgery was a failure to control bleeding, regardless of whether embolisation was repeated or not.22,35,38 Other indications reported include pain, acute coronary syndrome and increased free fluid on CT.6,38 RAE during a hospital stay may also be more frequent in patients later requiring nephrectomy due to more severe renal injuries.9 Still, timely nephrectomy remains an important last resort despite advancements in RAE.
Adjunctive endoscopic procedures such as retrograde ureteric stent placement were used in patients with urosepsis, symptomatic ureteral clot obstruction or significant urine extravasation on subsequent CT (3–5 days post-procedure) in patients with high-grade blunt renal trauma.9,12,22,23,38 The effectiveness of adjunctive stenting is currently unknown, but this subgroup of patients may represent those who have more severe injuries and are therefore at higher risk of poor long-term functional renal outcomes. With the current evidence available, patients with radiological markers of urinomas or urinary extravasation, lower urinary tract symptoms or uroseptic features may therefore require more consistent or extended follow-up to rationalise the need for ureteric stenting.
Technical Considerations
Level of Embolisation
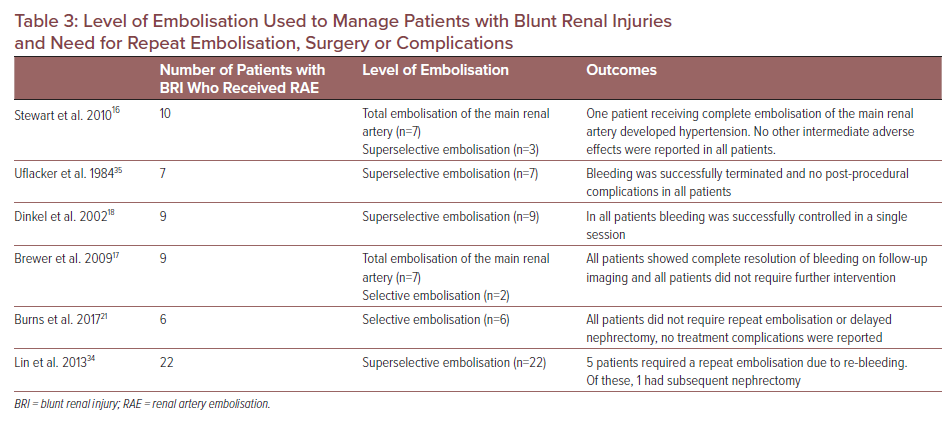
Embolisation of renal arteries leads to infarction downstream and eventual parenchymal death. Older direct catheterisation techniques often made sub-selecting smaller segmental branches impossible, leading to proximal embolisation and greater tissue loss.39,40 Therefore, as much renal parenchyma should be conserved by ‘selecting’ only the affected arteries at the most distal level to minimise loss of uninjured parenchyma. The preservation of renal parenchyma is an advantage of minimally invasive therapy compared to surgical intervention. Complete RAE of renal arteries was specified in only two studies, but the choice of renal artery total occlusion is usually reserved to achieve haemodynamic stability in polytrauma patients who are not fit for surgery due to associated injury or as an alternative to nephrectomy in cases not requiring surgical exploration.16,17
Newer selective and superselective methods allow for more directed localisation and catheterisation, as embolic material is deployed immediately proximal to the bleeding site using coaxial embolisation to preserve a greater proportion of nephrons, although most studies successfully controlled bleeding in a single session without further complications regardless of the level of embolisation (Table 3).29 Most tissue loss is therefore due to the original trauma itself.18 Neither superselective nor subselective embolisation are usually associated with a significant clinical reduction in renal function, but this depends on pre-procedural renal function, comorbidities and volume of contrast media used for embolisation.29,31,39–42 Superselective techniques may also limit delayed procedure-related complications, such as infection and post-traumatic hypertension.33
For main renal artery injuries, endovascular stenting may present a feasible alternative to embolisation to achieve renal revascularisation.43–47 Main renal artery dissections and/or luminal stenosis may benefit from endovascular stenting and covered stenting may be considered for proximal arterial rupture.48–50 Clinical factors to consider would include the presence of concurrent injuries as patients with stents would need to be anticoagulated and the presence of bleeding at other sites may limit stenting as an alternative.43,44
Embolic Agent
Multiple embolic agents are available for transcatheter RAE including particulates, sclerosants, glue and coils.
Metallic Coils
Metallic coils are desirable because of their accuracy and radiopacity.15 They are made of steel, titanium or platinum. The size of the coil must be adjusted to carefully match the vessel diameter to avoid poor placement, which was reported in one older study where there were two cases of extensive parenchymal loss due to incorrectly measured coil size.35,39
Metallic coils can be used to treat renal haemorrhage, AVF, pseudoaneurysms and arterio-calyceal fistulas, and multiple coils can be used in the same procedure.10,17,18 Selective embolisation of subsegmental branches with microcoils has also been used to limit the amount of embolised parenchyma.18
There are three main concerns with metallic coils. First, usually more than one coil is needed for adequate occlusion, increasing the time and cost of the procedure.46–50 Second, coils may be unstable and dislodged.35 There are two methods reported to ensure stability of the coils. The first and more commonly used method is to release the proximal aspect of the coil outside of the leaking vessel or into a nearby side branch with the remainder of the coil deployed in the vessel. The second method is to slightly oversize the coil to the vessel, relying on the outward radial force of the coil to secure it to the vessel wall so that the larger outer coil or coils act as a scaffolding onto which smaller coils can be deployed to produce the occluding effect.17 Newer detachable coils provide precision and control leading to accurate embolisation.
Particulate Embolic Agents
Absorbable Gelatine Sponges
Absorbable gelatine sponge embolisation may be useful to occlude small arterial aneurysms but may be related to aneurysm wall rupture owing to increased intra-aneurysmal pressure during embolus injection and passage through the arteriovenous fistula.35 One case report has illustrated success when using Gelfoam for unselective renal artery embolisation in a haemodynamically unstable patient with delayed partial renal artery canalisation.51 The authors of this case report attribute this success partially to the rapid haemostasis resulting from the mechanics of Gelfoam and partially due to the patient having small vessel collateralisation to the inferior pole of the kidney. Absorbable gelatine sponge particles may be easily fragmented and inadvertently ablate normal renal parenchyma. The effectiveness of the embolisation is also limited by distal movement of the absorbable gelatine sponge pledgets.52
Polyvinyl Alcohol
Polyvinyl alcohol (PVA) is similar to absorbable gelatine sponges in its manipulation, indications and delivery.35 However, PVA is more difficult to pass through the catheter in large particles and is not reabsorbable. PVA should be avoided in AVF as they can pass into venous circulation, potentially resulting in unintentional pulmonary embolism.35
Vascular Plugs
Vascular plugs are versatile and are ideal for embolising high-flow medium-to-large-sized vessels. They are self-expanding and exert adequate radial force to prevent migration even in a high-flow vessel. Vascular plugs can therefore be considered for main renal artery occlusion.53
Liquid Embolic Agents
Cyanoacrylates, such as N-butyl cyanoacrylate (NBCA) glue or Onyx® have a low viscosity and can be easily injected through small or tortuous catheters.46,48–50 NBCA glue may also be used in patients with haemodynamic instability or in patients with underlying coagulopathies as they achieve haemostasis faster than other embolic agents, but this was not specified in any studies using RAE to manage BRI. 40–50
Injury Characteristics
Mechanism of Injury
The most reported causes of injury were road traffic accidents involving cars, motorcycles or pedestrians.15–18,22 This was followed by falls and direct trauma.15–18
Presence of Concurrent Injuries
Blunt renal trauma can occur as an isolated injury or in a poly-trauma setting with injury to other non-urological organs. The most commonly reported concomitant injuries were to the abdominal viscera and thoracic structures, including the lungs, pleura or ribs, followed by pelvic fracture.17,18,22,54 Current European Association of Urology guidelines recommend renal exploration for high-grade renal trauma (AAST ≥3) and presence of concomitant intra-abdominal injuries as they can be managed in the same procedure. One study concluded that concomitant liver injury in combination with raised heart rate predicted the need for immediate intraoperative management in patients with high-grade BRI.7,55
Haemodynamic Status
RAE has a central role in the non-operative management of haemodynamically stable patients with blunt renal trauma.9–11,14 Persistent or delayed bleeding in a stable patient can be successfully managed with embolisation in most cases.2,15,54,56 Yet in patients with haemodynamic instability, defined as a systolic blood pressure of 90 mmHg or less, the role of embolisation is conflicting.15–17,22 Emergency laparotomy is traditionally favoured as these injuries are usually high grade and accompanied by other severe organ injuries which can also be managed during exploratory laparotomy.31,32,36
However, recent experience in Europe suggests that immediate angioembolisation by trained radiologists can be feasible regardless of patients’ haemodynamic status.9,38 Cases of isolated renal injury and haemodynamic instability can be safely managed with embolisation and in one study haemodynamic instability was not found to be a predictor of embolisation failure.9,10 In other studies, patients with haemodynamic instability were successfully controlled through embolisation and without the need for further surgical or radiological intervention.6,17,18,33
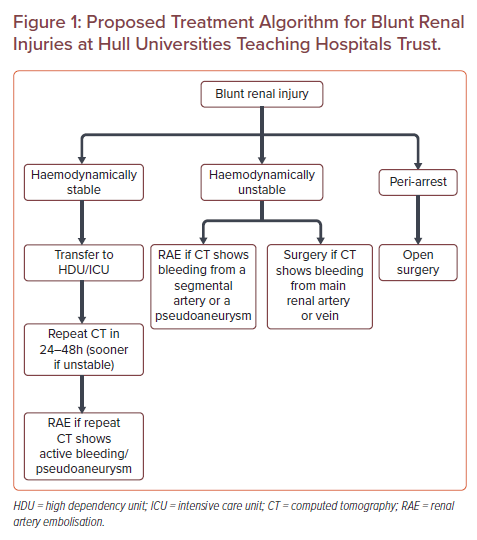
An exception may be during a peri-arrest situation, which is the period before or after a cardiac arrest. Clinically, the priority would be on immediate management of airway, breathing and circulation before proceeding to intervention. Surgical versus minimally invasive management of patients with BRIs would depend on local factors such as logistics and in-house availability of services. We have created a treatment algorithm for the management of BRIs that covers this period (Figure 1).
Anatomic Level of Injury
Knowledge of renal artery anatomy is important to accurately select the injured renal artery and preserve renal function. The renal arteries arise from the abdominal artery at L1–L2. Most patients have one renal artery feeding into each kidney, with the left renal artery being shorter than the right. A minority of patients have variant renal artery anatomy, which is usually an accessory renal artery that usually arise from the aorta but can also arise from any artery between the diaphragm and pelvis.57 Knowledge of variant anatomy is important as the actual site of contrast extravasation may be missed if arteriography is conducted on the main renal artery alone.
BRI can occur at any level of the renal vasculature. RAE was also used in patients with lesions at the level of the polar arteries, interlobar arteries, lobar branches, segmental branches and at the level of the main renal artery.15,17-18,21,27, 29-31,38,58
Complications
Post-embolisation Syndrome
Post-embolisation syndrome (PES) is the most common complication following RAE.42 Patients usually present with nausea, vomiting, fever and abdominal pain 1–3 days after embolisation.42 PES is usually self-limiting but prophylactic anti-inflammatories and anti-emetics may be given for symptomatic treatment.48 PES is more common when using liquid or particulate agents compared to coils, as they are more difficult to control under fluoroscopy.15 Only one study examined PES as a complication following embolisation for BRI and found that none of the patients experienced PES.18
Pain
Post-procedural pain usually lasts between 1–5 days.47 Patients may report post-procedural pain in the back or flank. The severity depends on the volume of parenchyma involved and is usually self-limiting.59 Patients should receive appropriate pre-, intra- and post-procedure analgesia.60
Arterial Hypertension
Transient increases in arterial blood pressure are a common post-procedural finding and can last for up to 24 hours, after which it resolves spontaneously and should not be a concern if complete occlusion is achieved.20,61 However, permanent hypertension may result from remnants of ischaemic (but not infarcted) tissue.47 One possible explanation could involve activation of the renin-angiotensin-aldosterone system secondary to either occlusion of the main renal artery, a branch from the main renal artery or AVF, or external compression of parenchyma by urine or blood.62
Renal Function
The risk factors for trauma-related renal impairment include pre-existing renal disease, age, having a single kidney and associated multi-organ failure.13 Blood clots may also lead to obstructive uropathy and after the resulting haematuria has resolved, renal function may return.42
Embolisation, particularly the less selective types, may also infarct healthy renal parenchyma and result in a decrease in renal function.56 One Chinese study found that RAE for Grade IV and V injuries led to worsened renal function after embolisation, but RAE was significantly more successful in preserving renal function compared to surgery.23 There was no difference in renal function at 6 months.23 Several other studies have found that renal function is not affected by RAE when serum creatinine was used as a crude indicator of renal function.18,23,35 In one study of 52 patients with high-grade renal trauma, RAE was not found to be a significant independent predictor of maximum serum creatinine rise compared to surgical management.35 Most patients in the study had BRIs, but those with penetrating trauma were also included in this study, which when taken into consideration with the small sample size, it is difficult to comment on whether the conclusions of the study are representative of the true effect.
The effects of iodinated contrast media on worsening renal function, particularly in hypotensive patients sustaining severe injuries, have been a cause for concern.38,63 The additional contrast needed does not increase the incidence of nephropathy regardless of renal injury grade, but patients should be adequately hydrated before embolisation.36,64
Haematuria
Haemorrhagic infarction may result in moderate haematuria following embolisation and usually resolves within 24–48 hours.20,37,47
Infection
Infection risk is low but there have been reported cases of post-RAE abscesses requiring percutaneous drainage.6,19,20,21 Interventional radiologists must be familiar with the patient’s past medical history, as reactivation of latent infections may also occur, evidenced by post-procedure CT scans showing air bubbles, although not all air bubbles are indicative of abscess formation.47 Some may correspond to normal aseptic infarction.48,49
Non-target Embolisation
Coil migration is a rare but serious complication of RAE. One study reported a dislodged microcoil into the lumbar artery in a patient due to catheter instability in an avulsed main renal artery, but no clinical consequences ensued.18 Endovascular snares can be used to retrieve migrated coils if that vessel cannot be safely killed.65 Advances in coil design, such as interlocking detachable coils and Guglielmi detachable coils allow for controlled deployment and easy retrieval if it is in an unfavourable position.18
Non-target embolisation of spinal arteries, lower extremity or bowel vessels can occur with particulate or liquid embolic agents. Adrenal artery non-target embolisation may lead to self-limiting transient hypertension or adrenal insufficiency and is treated conservatively.42 Correct catheter positioning, continuous monitoring during delivery of embolic agent and using occlusion balloon catheters can reduce the risk of non-target embolisation.60
Other Complications
Other complications that can arise with any endovascular procedure include access site haematoma, arterial thrombosis, arterial dissection, arterial rupture, distal site embolism, anaphylaxis, shock or volume overload.47
Discussion
BRIs are a common injury and although the use of embolisation to manage BRIs is becoming more widespread, the evidence base is still weak.
We examined the relevance of AAST grade on the outcomes for patients receiving embolisation. There is evidence to suggest that high-grade renal trauma (AAST ≥3) can be managed with embolisation. This is reflected in the present EAU guidelines which state that when RAE is included in the non-operative management pathways for patients with high-grade renal trauma, it can be successful in managing up to 94.9% of Grade III, 89% in Grade IV and 52% of Grade V injuries.9,10,25 Close examination of the patient’s CT to identify active contrast extravasation, pseudoaneurysm and arteriovenous fistulae may provide an accurate indication for the need for RAE.6,13,15,18,21,22,27 The patient’s CT should also be examined for polytrauma as concurrent intra-abdominal injuries may be managed intraoperatively.7 The patient’s clinical picture should also be considered, particularly their haemodynamic status. EAU guidelines suggest RAE in renal trauma patients who are haemodynamically stable, but there is some evidence to suggest that RAE is successful in patients with a systolic blood pressure <90 mmHg.7 In either case, the patient’s haemodynamic status should be considered with their overall clinical picture.6,9,10,17,18,33,38 More severe cases are usually accompanied by other severe organ injuries which may be managed concurrently during exploratory laparotomy. The risk of RAE failure should be balanced against the risk of secondary nephrectomy. The provision of 24-hour interventional radiology in major trauma centres may therefore play an integral role.
The choice of embolic agent for the management of BRI was also examined. Coils were the most commonly used embolic agent, probably because greater control can be achieved, particularly with microcoils under fluoroscopy, and the risk of post-embolisation syndrome that has been reported with particulate embolic agents due to reflux and non-target tissue ablation.15,17,18,20-22,29,31,34,35,39,58 Absorbable gelatine sponges (Gelfoam) were the second most commonly used embolic agent followed by PVA.17,18,21,31,34,35 These materials can be used as stand-alone agents, but combinations of microcoils with gelatine sponges or PVA have also been documented.17,18,31,34 As a general rule, Gelfoam is preferred for smaller pseudoaneurysms as it results in peripheral occlusion with minimal parenchymal loss, while coils in addition to Gelfoam are preferred for larger pseudoaneurysms.39 The choice of embolisation material depends on vascular anatomy, underlying pathology, size, flow pattern, material availability, experience and preference of the interventional radiologist.50 At our institution, microcoils are the most often used embolic agent and a combination of coils and gelatin sponges, such as coil/gel or foam/coil, are less commonly used to stop bleeding.
Finally, more common clinical complications are transient and self-limiting, such as post-embolisation syndrome, post-procedural pain, arterial hypertension, haematuria and renal impairment.18–20,22,23,35,47,50,59 Patients’ post-procedural status should be monitored appropriately so these complications can be managed. Appropriate monitoring will also alert clinicians to more serious complications such as intra-abdominal abscess formation, urosepsis, evidence suggestive of non-target embolisation or persistent bleeding which may warrant repeat embolisation or surgery.21,22,34,35,38,42
We have proposed a treatment algorithm in the management of patients with BRIs at a UK tertiary centre (Figure 1). In our unit, the management of BRIs depends on the haemodynamic stability of the patient and CT findings. Cases are discussed on a case-by-case basis with the on-call surgical team who are often on standby when RAE is performed on unstable patients. Our policy is for embolisation to be as superselective as possible. From our experience, the total procedure duration is approximately 20 minutes from common femoral needle access to successful embolisation, making RAE as quick or quicker than surgery depending on the complexity and number of vessels involved. Naturally for high-grade complex renal lesions with multiple segmental vessels involved procedure duration may increase and the benefits of RAE should be balanced against the benefits of surgical intervention. In the peri-arrest situation, the surgical teams are resident in hospital and so surgery is quicker. The interventional radiology team has a 30-minute call-out time.
This narrative review highlights institutional variation in practice and presents the available evidence surrounding the use of renal artery embolisation in the management of BRIs. While available evidence is promising for the use of RAE in the management of BRIs, this review article is limited in that only a few case series looked at renal artery embolisation in patients with BRIs.17,18,35 A formal meta-analysis was therefore not conducted because factors such as study design, mechanism of injury, definitions of clinical or technical success, study endpoints, reporting of complications, potential bias and the extent to which investigators controlled for confounding factors were too heterogeneous across the studies to enable comparison.
Conclusion
The evidence for renal artery embolisation in the management of renal trauma is so far promising, particularly with high-grade injuries and advances in embolisation techniques allowing for superselective methods and embolic agents on the order of microns. However, when considering patients with BRIs alone, most answers to the key clinical questions, such as optimal technique, CT indications, clinical status, comorbidities and complications are unknown. Retrospective reviews and case series do not provide robust evidence and sample size in these studies has been small. The question of operative versus non-operative management (including RAE) will remain unknown until the key clinical parameters as defined above, have been addressed by large, rigorous randomised controlled trials.
- Meng MV, Brandes SB, McAninch JW. Renal trauma: indications and techniques for surgical exploration. World J Urol 1999;17:71–7.
Crossref| PubMed - Santucci RA, McAninch JW, Safir M, et al. Validation of the American Association for the Surgery of Trauma organ injury severity scale for the kidney. J Trauma Inj Infect Crit Care 2001;50:195–200.
Crossref - Mani NBS, Kim L. The role of interventional radiology in urologic tract trauma. Semin Intervent Radiol 2011;28:415–23.
Crossref| PubMed - Schreyer HH, Uggowitzer MM, Ruppert-Kohlmayr A. Helical CT of the urinary organs. Eur Radiol 2002;12:575–91.
Crossref| PubMed - Kozar RA, Crandall M, Shanmuganathan K, et al. Organ injury scaling 2018 update: spleen, liver, and kidney. J Trauma Acute Care Surg 2018;85:1119–22.
Crossref| PubMed - Menaker J, Joseph B, Stein DM, Scalea TM. Angiointervention: high rates of failure following blunt renal injuries. World J Surg 2011;35:520–7.
Crossref| PubMed - Eueopean Association of Urology. EAU Guidelines on Urological Trauma. Amsterdam: European Association of Urology, 2022. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Urological-Trauma-2022_2022-03-24-104100_fwda.pdf (accessed 01 September 2022).
- Gor RA, Styskel BA, Li T, et al. Unexpected high rates of angiography and angioembolization for isolated low-grade renal trauma: results from a large, statewide, trauma database. Urology 2016;97:92–7.
Crossref| PubMed - Lanchon C, Fiard G, Arnoux V, et al. High grade blunt renal trauma: predictors of surgery and long-term outcomes of conservative management. A prospective single center study. J Urol 2016;195:106–11.
Crossref| PubMed - Shoobridge JJ, Bultitude MF, Koukounaras J, et al. A 9-year experience of renal injury at an Australian level 1 trauma centre. BJU Int 2013;112(Suppl 2):53–60.
Crossref| PubMed - van der Wilden GM, Velmahos GC, Joseph DK, et al. Successful nonoperative management of the most severe blunt renal injuries: a multicenter study of the research consortium of new England centers for trauma. JAMA Surg 2013;148:924–31.
Crossref| PubMed - Keihani S, Xu Y, Presson AP, et al. Contemporary management of high-grade renal trauma: results from the American Association for the Surgery of Trauma Genitourinary Trauma study. J Trauma Acute Care Surg 2018;84:418–25.
Crossref| PubMed - McGuire J, Bultitude MF, Davis P, et al. Predictors of outcome for blunt high grade renal injury treated with conservative intent. J Urol 2011;185:187–91.
Crossref| PubMed - Chow SJD, Thompson KJ, Hartman JF, Wright ML. A 10-year review of blunt renal artery injuries at an urban Level I Trauma Centre. Injury 2009;40:844–50.
Crossref| PubMed - Breyer BN, McAninch JW, Elliott SP, Master VA. Minimally invasive endovascular techniques to treat acute renal hemorrhage. J Urol 2008;179:2248–52.
Crossref| PubMed - Stewart AF, Brewer ME, Daley BJ, et al. Intermediate-term follow-up of patients treated with percutaneous embolization for grade 5 blunt renal trauma. J Trauma 2010;69:468–70.
Crossref| PubMed - Brewer ME, Strnad BT, Daley BJ, et al. Percutaneous embolization for the management of grade 5 renal trauma in hemodynamically unstable patients: initial experience. J Urol 2009;181:1737–41.
Crossref| PubMed - Dinkel HP, Danuser H, Triller J. Blunt renal trauma: minimally invasive management with microcatheter embolization experience in nine patients. Radiology 2002;223:723–30.
Crossref| PubMed - Fu CY, Wu SC, Chen RJ, et al. Evaluation of need for angioembolization in blunt renal injury: discontinuity of Gerota’s fascia has an increased probability of requiring angioembolization. Am J Surg 2010;199:154–9.
Crossref| PubMed - Aragona F, Pepe P, Patanã D, et al. Management of severe blunt renal trauma in adult patients: a 10-year retrospective review from an emergency hospital. BJU Int 2012;110:744–8.
Crossref| PubMed - Burns J, Brown M, Assi ZI, Ferguson EJ. Five-year retrospective review of blunt renal injuries at a level I trauma center. Am Surg 2017;83:148–56.
Crossref| PubMed - Baboudjian M, Gondran-Tellier B, Panayotopoulos P, et al. Factors predictive of selective angioembolization failure for moderate- to high-grade renal trauma: a French multi-institutional study. Eur Urol Focus 2022;8:253–8.
Crossref| PubMed - Xu H, Min X, Li Y, et al. A comparative study of conservation, endovascular embolization therapy, and surgery for blunt renal trauma 2020;25:e922802.
Crossref| PubMed - Hotaling JM, Sorensen MD, Smith TG, et al. Analysis of diagnostic angiography and angioembolization in the acute management of renal trauma using a national data set. J Urol 2011;185:1316–20.
Crossref| PubMed - Huber J, Pahernik S, Hallscheidt P, et al. Selective transarterial embolization for posttraumatic renal hemorrhage: a second try is worthwhile. J Urol 2011;185:1751–5.
Crossref| PubMed - Altman AL, Haas C, Dinchman KH, Spirnak JP. Selective nonoperative management of blunt grade 5 renal injury. J Urol 2000;164:27–30.
Crossref| PubMed - Figler BD, Malaeb BS, Voelzke B, et al. External validation of a substratification of the American Association for the Surgery of Trauma renal injury scale for grade 4 injuries. J Am Coll Surg 2013;217:924–8.
Crossref| PubMed - Glass AS, Appa AA, Kenfield SA, et al. Selective angioembolization for traumatic renal injuries: a survey on clinician practice. World J Urol 2014;32:821–7.
Crossref| PubMed - Chatziioannou A, Brountzos E, Primetis E, et al. Effects of superselective embolization for renal vascular injuries on renal parenchyma and function. Eur J Vasc Endovasc Surg 2004;28:201–6.
Crossref| PubMed - Saour M, Charbit J, Millet I, et al. Effect of renal angioembolization on post-traumatic acute kidney injury after high-grade renal trauma: a comparative study of 52 consecutive cases. Injury 2014;45:894–901.
Crossref| PubMed - Sarani B, Powell E, Taddeo J, et al. Contemporary comparison of surgical and interventional arteriography management of blunt renal injury. J Vasc Interv Radiol 2011;22:723–8.
Crossref| PubMed - Aldiwani M, Georgiades F, Omar I, et al. Traumatic renal injury in a UK major trauma centre – current management strategies and the role of early re-imaging. BJU Int 2019;124:672–8.
Crossref| PubMed - Hagiwara A, Sakaki S, Goto H, et al. The role of interventional radiology in the management of blunt renal injury: a practical protocol. J Trauma 2001;51:526–31.
Crossref| PubMed - Lin WC, Lin CH, Chen JH, et al. Computed tomographic imaging in determining the need of embolization for high-grade blunt renal injury. J Trauma Acute Care Surg 2013;74:230–5.
Crossref| PubMed - Uflacker R, Paolini RM, Lima S. Management of traumatic hematuria by selective renal artery embolization. J Urol 1984;132:662–7.
Crossref| PubMed - Husmann DA, Gilling PJ, Perry MO, et al. Major renal lacerations with a devitalized fragment following blunt abdominal trauma: a comparison between nonoperative (expectant) versus surgical management. J Urol 1993;150:1774–7.
Crossref| PubMed - Sugihara T, Yasunaga H, Horiguchi H, et al. Management trends, angioembolization performance and multiorgan injury indicators of renal trauma from Japanese administrative claims database. Int J Urol 2012;19:559–633.
Crossref| PubMed - Long JA, Fiard G, Descotes JL, et al. High-grade renal injury: non-operative management of urinary extravasation and prediction of long-term outcomes. BJU Int 2013;111:E249–55.
Crossref| PubMed - Corr P, Hacking G. Embolization in traumatic intrarenal vascular injuries. Clin Radiol 1991;43:262–4.
Crossref| PubMed - Fisher RG, Ben-Menachem Y, Whigham C. Stab wounds of the renal artery branches: angiographic diagnosis and treatment by embolization. AJR Am J Roentgenol 1989;152:1231–5.
Crossref| PubMed - Poulakis V, Ferakis N, Becht E, et al. Treatment of renal-vascular injury by transcatheter embolization: immediate and long-term effects on renal function. J Endourol 2006;20:405–9.
Crossref| PubMed - Ginat DT, Saad WEA, Turba UC. Transcatheter renal artery embolization: clinical applications and techniques. Tech Vasc Interv Radiol 2009;12:224–39.
Crossref| PubMed - Jahangiri Y, Ashwell Z, Farsad K. Percutaneous renal artery revascularization after prolonged ischemia secondary to blunt trauma: pooled cohort analysis. Diagn Interv Radiol 2017;23:371–8.
Crossref| PubMed - Lim KH, Ryeom HK, Park J. Endovascular treatment of renal arterial perforation after blunt trauma: case report. Int J Surg Case Rep 2018;42:208–11.
Crossref| PubMed - Dobrilovic N, Bennett S, Smith C, et al. Traumatic renal artery dissection identified with dynamic helical computed tomography. J Vasc Surg 2001;34:562–4.
Crossref| PubMed - Lee JT, White RA. Endovascular management of blunt traumatic renal artery dissection. J Endovasc Ther 2002;9:354–8.
Crossref| PubMed - Loffroy R, Chevallier O, Gehin S, et al. Endovascular management of arterial injuries after blunt or iatrogenic renal trauma. Quant Imaging Med Surg 2017;7:434–42.
Crossref| PubMed - Loffroy R, Rao P, Kwak BK, et al. Transcatheter arterial embolization in patients with kidney diseases: an overview of the technical aspects and clinical indications. Korean J Radiol 2010;11:257–68.
Crossref| PubMed - Loffroy R, Guiu B, Lambert A, et al. Management of post-biopsy renal allograft arteriovenous fistulas with selective arterial embolization: immediate and long-term outcomes. Clin Radiol 2008;63:657–65.
Crossref| PubMed - Loffroy R, Guiu B, Cercueil JP, Krause D. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol 2009;7:250–63.
Crossref| PubMed - Jairam A, King B, Berman Z, Rivera-Sanfeliz G. Non-permanent transcatheter proximal renal artery embolization for a grade 5 renal injury with delayed recanalization and preserved renal parenchymal enhancement. J Trauma Inj 2021;34:198–202.
Crossref - Uflacker R. Transcatheter embolisation of arterial aneurysms. Br J Radiol 1986;59:317–24.
Crossref| PubMed - Ramakrishnan S. Vascular plugs – a key companion to interventionists – ‘Just Plug it’. Indian Heart J 2015;67:399–405.
Crossref| PubMed - Hagiwara A, Murata A, Matsuda T, et al. The usefulness of transcatheter arterial embolization for patients with blunt polytrauma showing transient response to fluid resuscitation. J Trauma Inj Infect Crit Care 2004;57:271–6.
Crossref| PubMed - May AM, Darwish O, Dang B, et al. Successful nonoperative management of high-grade blunt renal injuries. Adv Urol 2016;2016:3568076.
Crossref| PubMed - Mohsen T, El-Assmy A, El-Diasty T. Long-term functional and morphological effects of transcatheter arterial embolization of traumatic renal vascular injury. BJU Int 2008;101:473–7.
Crossref| PubMed - Horan F. Gray’s Anatomy: the anatomical basis of clinical practice. J Bone Joint Surg Br 2009;91–B:983.
Crossref - Lopera JE, Suri R, Kroma G, et al. Traumatic occlusion and dissection of the main renal artery: endovascular treatment. J Vasc Interv Radiol 2011;22:1570–4.
Crossref| PubMed - Pretorius R, Vlok S, van der Merwe A, et al. Renal artery embolisation: indications and utilisation at Tygerberg Hospital. S Afr J Surg 2019;57:33–9.
Crossref| PubMed - Ramaswamy RS, Darcy MD. Arterial embolization for the treatment of renal masses and traumatic renal injuries. Tech Vasc Interv Radiol 2016;19:203–10.
Crossref| PubMed - McAninch JW, Carroll PR, Klosterman PW, et al. Renal reconstruction after injury. J Urol 1991;145:932–7.
Crossref| PubMed - Wein AJ, Kavoussi LR, Partin AW, Peters CA. Campbell-Walsh Urology. 11th ed. Amsterdam: Elsevier, 2015; 2.
- Thomsen HS, Marcos SK, Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). In which patients should serum creatinine be measured before iodinated contrast medium administration? Eur Radiol 2005;15:749–54.
Crossref| PubMed - Ierardi AM, Duka E, Lucchina N, et al. The role of interventional radiology in abdominopelvic trauma. Br J Radiol 2016;89:20150866.
Crossref| PubMed - Schwartz MJ, Smith EB, Trost DW, Vaughan ED. Renal artery embolization: clinical indications and experience from over 100 cases. BJU Int 2007;99:881–6.
Crossref| PubMed