Grigorios Korosoglou, Sorin Giusca, Martin Andrassy, Michael Lichtenberg
|
Abstract Keywords Disclosure: The authors have no conflicts of interest to declare. Received: Accepted: Published online: Citation: Vascular & Endovascular Review 2019:2(1):12–8. DOI: https://doi.org/10.15420/ver.2018.16.2 Correspondence Details: Grigorios Korosoglou, GRN Academic Teaching Hospital Weinheim, Department of Cardiology and Vascular Medicine, Roentgenstrasse 1, D-69469, Weinheim, Germany. E: gkorosoglou@hotmail.com Open Access:
This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly. |
The prevalence of peripheral artery disease (PAD) has been rising consistently over the past few decades, along with increasing rates of type 2 diabetes. More than 200 million people are estimated to be affected by PAD worldwide.1 Despite aggressive modifications of lifestyle and risk factors, and advances in the pharmacological management of patients with PAD using antiplatelet agents, statins, cilostazol, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers and low-dose anticoagulation with rivaroxaban, patients with PAD frequently require invasive procedures to improve the symptoms of claudication and prevent tissue damage or loss in those with critical limb ischaemia (CLI).2
Due to recent technological advances in materials and devices for the endovascular treatment of PAD, a minimally invasive percutaneous approach is considered a first-choice strategy for the treatment of symptomatic patients and is given preference over older surgical options. According to current guidelines, endovascular therapy is the most favoured option for infrainguinal stenotic or occlusive lesions <25 cm, whereas open surgery may be associated with better long-term patency, especially when using saphenous vein grafts in patients with long occlusive lesions (>25 cm) that cross the knee.3Commonly used techniques include plain balloon angioplasty, drug-coated balloon (DCB) angioplasty, bare metal stents, drug-eluting stents and stent grafts for vessel perforation. Such endovascular approaches have been successfully used to alleviate symptoms in patients with claudication and have been associated with limb salvage in patients with CLI.4–6
In many cases, such ‘basic’ approaches may be compromised by severe calcification. Calcification may be the reason for a poor primary outcome due to early recoil or extensive flow-limiting dissections after high-pressure angioplasty, culminating in the need for bailout stent placement.7 Despite the most recent self-expandable nitinol stent technology, rates of re-stenosis may be high, resulting in primary patency rates of <50% for bare metal stents and <70% for drug-eluting stents at 5-year follow-up.8 Even with dedicated stent devices, stent fractures may occur at sites of extensive movement and flexion as in the popliteal artery, resulting in stent thrombosis and subsequent occlusion.9
With conventional open surgery, 5-year patency rates of more than 80% have been reported with saphenous vein grafts compared with 67% when using prosthetic conduits.10 Although no randomised trials are currently available for a head-to-head comparison of endovascular versus surgical treatment for long and calcified femoropopliteal lesions, it may be assumed that alternative treatment strategies will be needed to improve long-term patency rates with endovascular treatment. We need more advanced plaque modification techniques which would permit lesion preparation by endovascular debulking of calcified and fibrotic tissue. After performing adequate lesion preparation by atherectomy devices, the application of local pharmacological treatment with DCB and without stent placement would be desirable. Over the past few years, this approach had proved beneficial for the treatment of complex femoropopliteal lesions.11
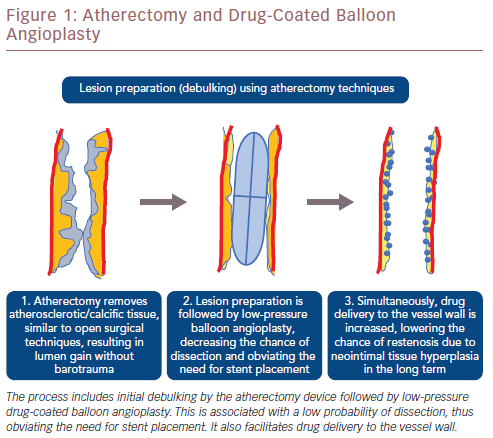
Modalities of atherectomy can result in the removal of plaque and calcified tissue equivalent to that achieved by surgical techniques as well as minimally invasive percutaneous treatment. Atherectomy permits removal of calcified and fibrotic tissue rather than pressing it against the arterial wall. This provides luminal gain without barotrauma, reduces the risk of dissection in the acute setting and neointimal hyperplasia in the long term. Atherectomy is subsequently combined with low-pressure balloon angioplasty, minimising the likelihood of dissection and the need for stent placement. This approach concurs with the notion of ‘leaving no metal behind’, especially when treating mobile peripheral vessel segments. Avoiding stents in such vessel segments may improve long-term patency and facilitate future reinterventions, while still permitting the option of future surgical procedures. Even in densely calcified vessels that require stent placement, incomplete or eccentric stent expansion will be avoided because the lesions will have been adequately prepared beforehand. Despite the use of atherectomy devices, spotted stent implantation may be required with persistent recoil after atherectomy and balloon angioplasty, or persistent flow-limiting dissection. In addition, the placement of a coated stent may be necessary as a bailout option after vessel perforation due to the atherectomy device. A schematic illustration of each step of atherectomy and DCB angioplasty is shown in Figure 1.
Four methods of atherectomy have been used for the treatment of infrainguinal disease:
- Directional atherectomy.
- Rotational atherectomy.
- Orbital atherectomy.
- Hybrid atherectomy.
Each of these devices has unique features, with advantages and disadvantages with respect to lesion characteristics. The safety profile and efficacy of these devices has not yet been compared. Generally, atherectomy can be used for a broad spectrum of lesion characteristics although relative contraindications are subintimal crossing of the lesion and a vessel diameter smaller than that indicated for the respective device in the instructions for use. It should be noted that devices primarily designed for thrombectomy, such as Angiojet™ (Boston Scientific), Penumbra™ (Penumbra) or photoablation, such as excimer laser using the Turbo-Elite and Turbo-Power system (Spectranetics), are not discussed in the present article as the procedures do not involve atherectomy in the traditional sense of cutting or removing tissue.
Directional Atherectomy
Carbide rotating cutter blades are used to cut and remove atherosclerotic tissue. Plaque is removed by the cutter device, which can be directly guided to the target lesion and rotated in the preferred direction. Directional atherectomy can tackle eccentric lesions more effectively than concentric ones. However, several passages are needed to achieve sufficient removal of atherosclerotic tissue. The resected tissue is collected in a nose cone, which must be emptied after only a few passages. As no aspiration mechanism is involved when using these devices, plaque embolisation is a concern. The use of a distal protection filter is advisable, especially when addressing heavily calcified lesions.
To date, three devices are available for directional atherectomy: the SilverHawk™, TurboHawk™ and the more recent HawkOne™ (all Medtronic), which can be used to treat peripheral lesions in vessels with a diameter of 1.5–7.0 mm.12 SilverHawk has one inner cutting blade and the TurboHawk has four. The four blades allow the device to remove a larger quantity of tissue per passage. This is an advantage when there is strongly calcified lesions. The HawkOne device has a single inner blade, which provides a better crossing profile and it has a distal flushing tool to simplify the cleaning process and allow more efficient excision.
The safety and efficacy of directional atherectomy devices have been investigated in several prospective multicentre studies.13–18 The first independently adjudicated prospective multicentre trial investigating the safety and efficacy of directional atherectomy was the Determination of Effectiveness of SilverHawk Peripheral Plaque Excision for the Treatment of Infrainguinal Vessels (DEFINITIVE LE). It involved 800 patients with claudication symptoms or CLI who were enrolled at 50 sites in the US and Europe.14 The device was associated with a success rate of 89% and bailout stenting rates of 3.2%. However, rates of distal embolisation, dissection, perforation and acute vessel occlusion were 3.8%, 2.3%, 5.3% and 2.0%, respectively. The mean lesion length was relatively low (7.5 cm in patients with claudication and 7.2 cm in patients with CLI), and only 40% of the lesions were calcified. In addition, the bailout stent rate was 3.2%, which was lower than that registered in preceding smaller studies.13 At the 2-month follow-up, the primary patency rate was 78% in patents with claudication, whereas 95% of patients with CLI were free of unplanned major amputation.
Further outcome data for patient subgroups with popliteal and infrapopliteal lesions from the DEFINITIVE LE study have been reported recently.15,16,19 The safety and efficacy of the SilverHawk and TurboHawk devices with the use of distal filter protection was reported in the DEFINITIVE Ca trial, in which 168 lesions with moderate to severe calcification were treated.17 The primary endpoint of effectiveness, defined as ≤50% residual stenosis, was achieved in 92% of the lesions, whereas the filter devices prevented embolic events in 97.5% (n=119) of 122 cases where excised tissue was captured.
More recently, the DEFINITIVE AR trial sought to compare upfront directional atherectomy combined with DCB versus a DCB-only strategy in a randomised manner in patients with femoropopliteal disease.18 Combined treatment with atherectomy and DCB was effective and safe, but no added value was observed in comparison with a DCB-only strategy at 1-year follow-up. The authors concluded that larger prospective studies will be needed to address this question. More recently, directional atherectomy in combination with DCB was used in 30 consecutive patients with common femoral artery stenosis or occlusion.20 In this single-centre study, the procedural success was 100%. The authors noted a low rate of stenting (10%) and a very high patency rate of 97% at the follow-up investigation after 1 year. The DiRectional AthErectomy + Drug-CoAted BaLloon to Treat Long, CalcifIed FemoropopliTeal ArterY Lesions (REALITY) study is a prospective, single-arm multicentre study that will enrol 150 patients to evaluate the adjunctive use of atherectomy using the TurboHawk and HawkOne devices and DCB treatment in long and moderate-to-heavily calcified femoropopliteal lesions. In addition, the current Atherectomy and Drug-Coated Balloon Angioplasty in Treatment of Long Infrapopliteal Lesions (ADCAT) trial compares the performance of directional atherectomy and DCB versus a DCB-only treatment strategy in long de novo infrapopliteal lesions in a prospective randomised setting.
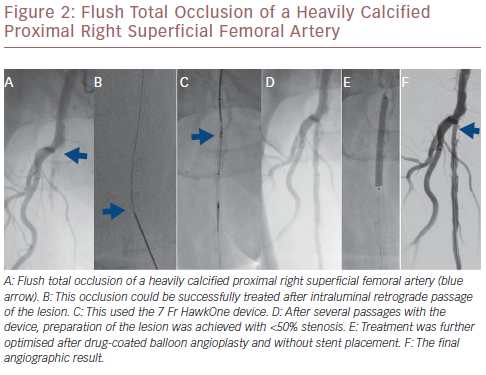
The Pantheris system combines optical coherence tomography (OCT) with peripheral atherectomy. OCT is used in this context to facilitate intraluminal recanalisation of the occluded peripheral arteries.21 The safety and effectiveness of this device was recently demonstrated in the Evaluation of the Pantheris Atherectomy System (VISION) trial where a technical success rate of 97% was achieved with a diameter stenosis reduction from 78.7% at baseline to 30.3% after atherectomy alone and with a low rate of peri-procedural complications, such as perforation (0%), catheter-related dissection (0.5%) and embolisation (2%).21 Figure 2 illustrates a flush total occlusion of the heavily calcified proximal right superficial femoral artery (SFA), which was successfully tackled after intraluminal retrograde passage of the lesion.
Rotational Atherectomy
Atherosclerotic tissue is concentrically excised using specially designed rotating tips or burrs. The luminal gain matches the size of the tip. Some of the devices are equipped with additional cutting blades to maximise debulking. The systems currently available for rotational atherectomy are the Rotarex®S system (Straub Medical), the Jetstream Atherectomy device (Boston Scientific) and the peripheral Rotablator system (Boston Scientific). The Rotarex S is a purely mechanical thrombectomy and atherectomy device, consisting of an external drive system which is connected to the Rotarex S catheter system by a magnetic clutch. A helix inside the catheter transmits the rotation from the drive system to the catheter head, which can rotate up to 10,000 rpm, creating a powerful negative suction force, facilitating the collection of fragmented thrombi and tissue material into an external bag following the Archimedes’ principle. It is available in 6 Fr, 8 Fr and 10 Fr sizes and is inserted over a dedicated guidewire. The device can be safely used in vessels of 3–8-mm diameter.
Jetstream employs a 7 Fr platform and is available with two types of catheters, one equipped with a single set of front cutting blades and one with a second set of larger blades to increase the capability of upfront debulking. Continuous aspiration and active removal of the excised tissue and thrombus is ensured. This device could be especially useful in partly thrombotic lesions with subacute arterial occlusions. Data concerning the use of the Rotablator in peripheral arteries is limited. However, Jetstream was tested in a multicentre single-arm study involving 172 patients with infrainguinal lesions, yielding a device success rate of 99% and a 74% rate of freedom from target vessel revascularisation at 1-year follow-up.22,23 Using the Jetstream device, stent placement was performed in 7% of the lesions, whereas the primary and secondary duplex-documented patency rates were 61.8% and 81.3%, respectively.22
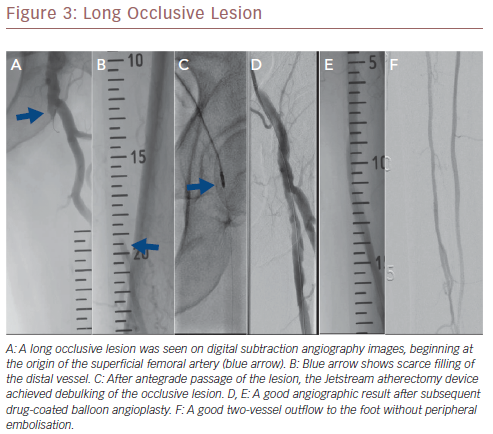
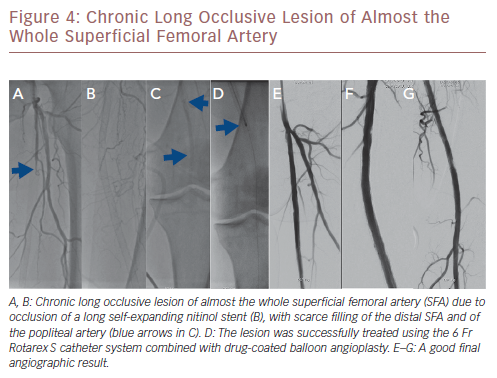
More recently, a relatively large retrospective study reported on long-term outcomes after atherectomy combined with angioplasty and provisional stenting versus angioplasty-only in 167 patients with common femoral artery disease.24 Patients in the combined atherectomy and angioplasty group had a significantly higher patency rate compared with the angioplasty group, implying the added value of atherectomy in these patients. In another study on the safety and efficacy of the Jetstream device in a real-world setting in 241 patients with femoropopliteal disease, the Jetstream achieved a high procedural success of 98.3% with low procedural complication rates (1% distal embolisation), and a relatively high rate of freedom from target lesion revascularisation (82%) after 1 year.25 However, higher rates of distal embolisation (8%) were reported in another similar study, which could be reduced to 2% using a distal protection filter.26Figure 3 shows a chronic long occlusive lesion beginning at the level of the proximal SFA with scarce filling of the distal SFA. Figure 4 shows a chronic long occlusive lesion beginning at the proximal SFA due to occlusion of a long self-expanding nitinol stent.
Orbital Atherectomy
An eccentrically mounted diamond-coated crown that orbits 360 degrees within the vessel is used for circumferential plaque removal. This allows calcified tissue to be removed without causing vessel wall trauma. In contrast to rotational atherectomy, which is limited by the size of the catheter tip or burr size, the debulked area can be increased by raising the rotational speed of the eccentrically mounted crown. Orbital atherectomy does not permit aspiration. Like rotablation, distal embolisation due to the small particles produced by rapid crown rotation cannot be ruled out. The use of a distal protection device is advisable. Currently available orbital atherectomy devices include the CSI Stealth 360 and Diamondback 360 Orbital atherectomy systems (both from Cardiovascular Systems). Several clinical studies have confirmed that orbital atherectomy permits low-pressure balloon angioplasty after successful debulking, which was verified by intravascular imaging.27
Orbital atherectomy has been evaluated in several single-arm clinical trials including Orbital Atherectomy System for the Treatment of Peripheral Vascular Stenosis (OASIS), the COroNary CT Angiography Evaluation For Clinical Outcomes: An InteRnational Multicenter Registry (CONFIRM) and COMPLIANCE 360°, which together included more than 4,000 patients.28–31 In the OASIS trial, 124 patients with 201 infrapopliteal lesions were treated with orbital atherectomy and the results were entered in a single-arm multicentre registry; 111 (55%) lesions were considered heavily calcified.28 Procedural success was achieved in 90% of the treated lesions, whereas procedural complications were noted in five patients (4%). At follow-up at 6 months, no patient required bypass surgery or unplanned amputation. An improvement on the Rutherford classification was observed in 78% of patients.28
In the CONFIRM registry trial, 4,766 lesions were treated with orbital atherectomy in 3,135 patients.29 In this large registry, orbital atherectomy combined with balloon angioplasty effectively reduced the degree of lesion lumen narrowing from 88% to 10%. Despite the relatively high rate of either moderately or severely calcified lesions (81%), the rate of stenting was relatively low (5.7%). In a more recent subsection analysis of the CONFIRM registries, the safety and efficacy of the technique was confirmed for the treatment of common femoral, iliac and even deep femoral artery lesions.32–34 The smaller COMPLIANCE 360° trial comprised 50 patients with heavily calcified femoropopliteal lesions who underwent orbital atherectomy plus angioplasty versus angioplasty alone.31 At 1-year follow-up, patency rates were similar (~80%) in both patient groups, despite significantly higher rates of bailout stenting in patients undergoing angioplasty alone (5.3% versus 77.8%; p<0.001). In another similar single-centre trial, the value of orbital atherectomy combined with DCB angioplasty was evaluated for the treatment of calcified femoropopliteal lesions in 89 patients.35 Despite the higher degree of calcification in patients who underwent atherectomy and DCB compared with those who underwent DCB alone (83% versus 42%; p<0.001), the rate of bailout stenting was lower in the combined therapy group (18% versus 39%; p=0.01). Furthermore, despite more complex lesions in the combined therapy group, patency rates were similar in both.
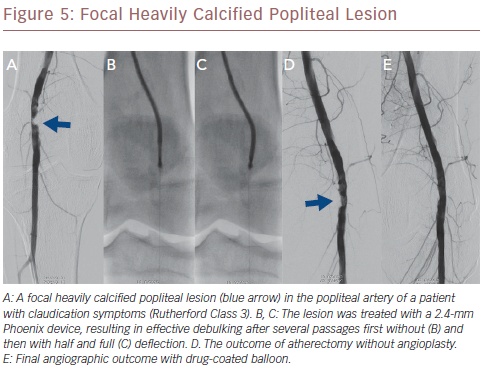
Hybrid atherectomy combines features of directional and rotational atherectomy. The advantage is that the cutter device can be directly guided to the target lesions, while the high rotational speed of the cutter device enables continuous aspiration of the excised tissue. This modality was introduced with the Phoenix device (Philips Volcano), which was given Food and Drug Administration (FDA) approval for peripheral use in the US. The device is available in 1.8-mm, 2.2-mm and 2.4-mm sizes (which are compatible with 5 Fr, 6 Fr and 7 Fr sheaths, respectively), and with a 0.014-inch guidewire for the treatment of femoropopliteal and below-the-knee lesions. While the 1.8-mm and 2.2-mm catheter sizes serve as regular rotational atherectomy devices, the 2.4-mm catheter possesses a deflecting tip, thus facilitating a hybrid atherectomy modus.
The Phoenix device consists of two main components: a long, double lumen catheter with a metallic front cutter at its distal tip, and the battery-powered handle device for atherectomy. The handle device is disposable and does not require capital equipment. The cutter is rotated at high speed (10,000–12,000 rpm) which, in accordance with Archimedes’ principle, creates a strong suction force and allows continuous aspiration of the fragmented excised tissue into an external bag. Atherectomy can be performed with this device in vessel diameters of 2.5–7.0 mm.
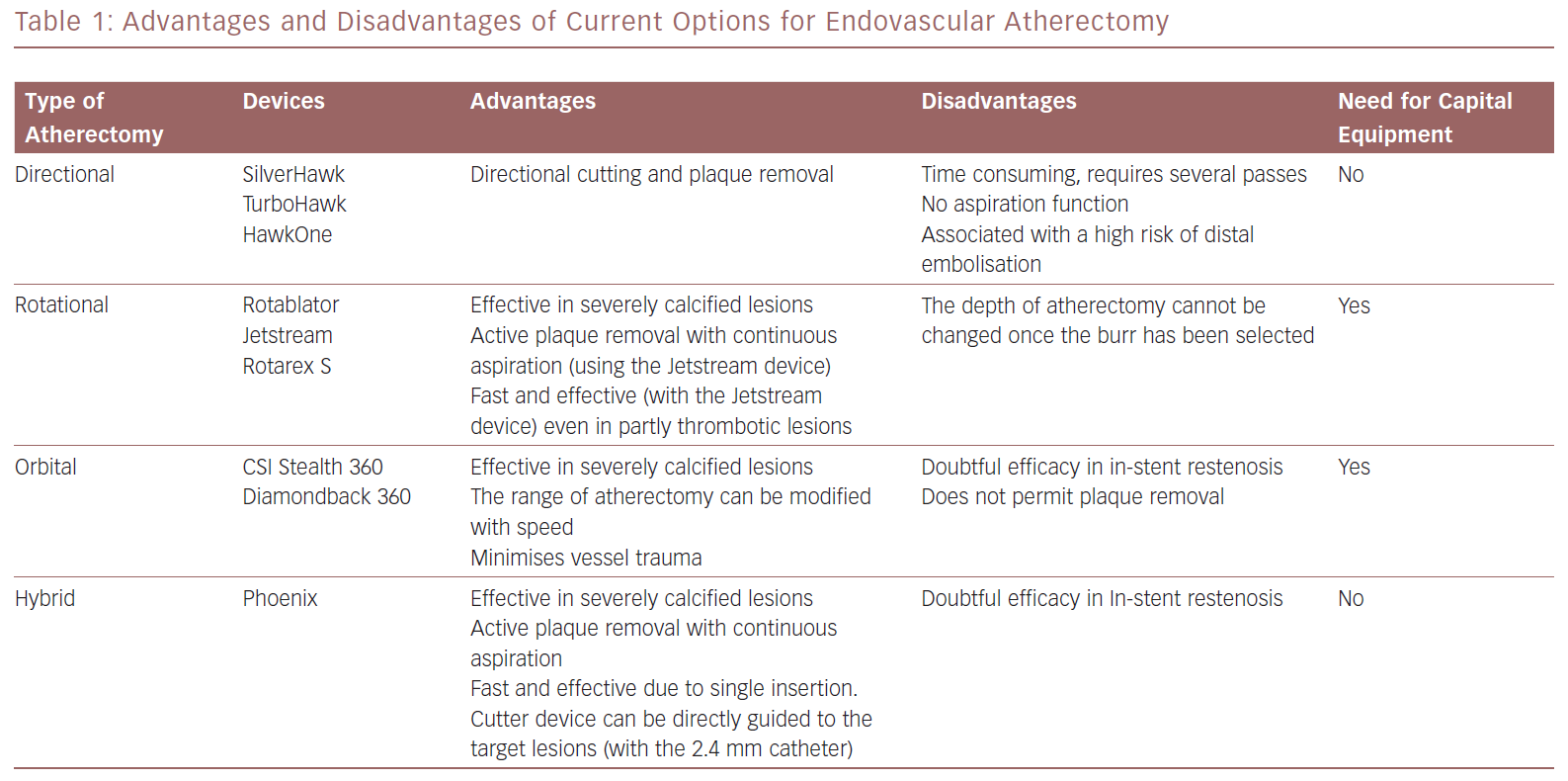
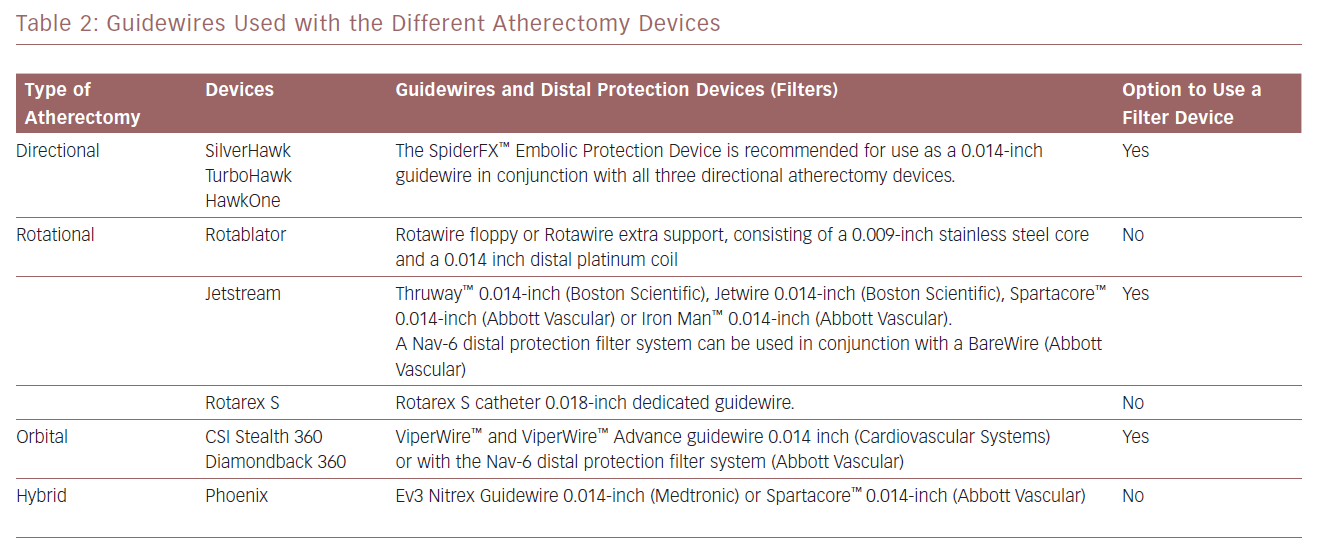
The advantages and disadvantages of the atherectomy devices discussed in this article are summarised in Table 1. Information on the guidewires and distal protection systems that can be used with these devices is provided in Table 2.
Common Femoral Artery Disease
For patients with common femoral artery lesions, endarterectomy and patch angioplasty is an established method of treatment with good long-term results.37,38 However, more recent studies have pointed to non-negligible complication and infection rates with this procedure, and a registry including 1,843 patients who underwent surgical endarterectomy demonstrated a high rate of 30-day mortality (3.4%), wound infections (8%) and need for further operations (10.2%).39–41 Therefore, in patients with common femoral artery disease, surgical endarterectomy may be considered the method of choice especially in young patients with calcified, de novo lesions if they are at low risk for surgery. Endovascular techniques may be a useful alternative option in patients with comorbidities, those who are unfit for surgery and in patients prone to develop wound infections after surgery, including patients with obesity, diabetes or a compromised immune response. In addition, hybrid procedures, including common femoral endarterectomy and patch angioplasty followed by endovascular atherectomy of the superficial femoral artery, may be useful in patients with combined common and superficial femoral artery occlusive disease.
Future Perspectives
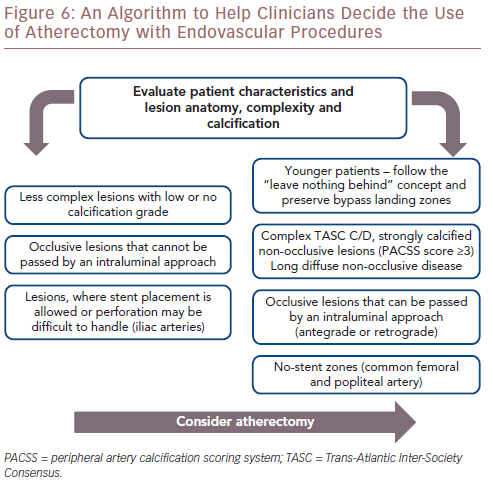
A large number of FDA-approved atherectomy devices are currently available. However, published reports concerning their comparative safety and efficacy do not exist. Due to additional procedural costs, and capital equipment costs with some devices, randomised multicentre studies are necessary to determine the added value of this modality. Considering the safety and long-term effectiveness of most atherectomy devices, it is conceivable that the use of atherectomy will be increase in the next few years, especially in anatomical no-stent zones in the common femoral and popliteal arteries, in younger patients and in complex, long and calcified Trans-Atlantic Inter-Society Consensus C/D lesions. An algorithm designed to help clinicians to decide whether to use atherectomy with endovascular procedures is provided in Figure 6.
Atherectomy promises to be a procedure that leaves no metal behind, especially in the presence of mobile arterial segments, and retains the possibility of future surgical options, particularly in younger patients without cardiopulmonary comorbidities. From the practical point of view, atherectomy devices will probably be readily accepted by interventional cardiologists familiar with coronary rotablation and by interventionalists who have performed rotational thrombectomy procedures. Considering the availability of diverse atherectomy devices, it would be advisable to first gain expertise in the use of a single device, with due attention to patient selection and lesion characteristics.
Conclusion
Endovascular atherectomy has emerged as a novel technique for plaque removal in PAD, offering the benefits of tissue removal equivalent to that of traditional surgical endarterectomy as well as the advantages of minimally invasive percutaneous treatment. Plaque is actively excised and simultaneously aspirated or fragmented rather than pressed against the arterial wall. This provides luminal gain without vessel wall trauma and enhances the chances of homogeneous balloon expansion during subsequent angioplasty at low pressures. The risk of dissection is reduced, possibly obviating the need for stent placement and reducing the risk of neointimal hyperplasia in the long term.
References
- Gallino A, Aboyans V, Diehm C, et al. Non-coronary atherosclerosis. Eur Heart J 2014;35:1112–9
Crossref| PubMed - Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45:S5–67
Crossref| PubMed - Aboyans V, Ricco J-B, Bartelink M-LEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816
Crossref| PubMed - Greenhalgh RM, Belch JJF, Brown LC, et al. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: results from two randomised trials for stenotic femoropopliteal and aortoiliac arterial disease. Eur J Vasc Endovasc Surg 2008;36:680–8
Crossref| PubMed - Fakhry F, Spronk S, van der Laan L, et al. Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication: a randomized clinical trial. JAMA 2015;314:1936–44
Crossref| PubMed - Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: from 2003–2011. J Am Coll Cardiol 2016; 67:1901–13
Crossref| PubMed - Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation 1992;86:64–70
Crossref| PubMed - Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation 2016;133:1472–83
Crossref| PubMed - Cambiaghi T, Spertino A, Bertoglio L, Chiesa R. Fracture of a Supera interwoven Nitinol stent after treatment of popliteal artery stenosis. J Endovasc Ther 2017;24:447–9
Crossref| PubMed - Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg 2004;27:357–62
Crossref| PubMed - Korosoglou G, Lichtenberg M, Celik S, et al. The evolving role of drug-coated balloons for the treatment of complex femoropopliteal lesions. J Cardiovasc Surg (Torino) 2018;59:51–9
Crossref| PubMed - Akkus NI, Abdulbaki A, Jimenez E, Tandon N. Atherectomy devices: technology update. Med Devices Auckl 2014;8:1–10
Crossref| PubMed - Shammas NW, Coiner D, Shammas GA, et al. Percutaneous lower-extremity arterial interventions with primary balloon angioplasty versus Silverhawk atherectomy and adjunctive balloon angioplasty: randomized trial. J Vasc Interv Radiol JVIR 2011;22:1223–8
Crossref| PubMed - McKinsey JF, Zeller T, Rocha-Singh KJ, et al. Lower extremity revascularization using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study. JACC Cardiovasc Interv 2014;7:923–33
Crossref| PubMed - Garcia LA, Jaff MR, Rocha-Singh KJ, et al. A comparison of clinical outcomes for diabetic and nondiabetic patients following directional atherectomy in the DEFINITIVE LE claudicant cohort. J Endovasc Ther 2015;22:701–11
Crossref| PubMed - Rastan A, McKinsey JF, Garcia LA, et al. One-year outcomes following directional atherectomy of infrapopliteal artery lesions: subgroup results of the prospective, multicenter DEFINITIVE LE trial. J Endovasc Ther 2015;22:839–46
Crossref| PubMed - Roberts D, Niazi K, Miller W, et al. Effective endovascular treatment of calcified femoropopliteal disease with directional atherectomy and distal embolic protection: final results of the DEFINITIVE Ca++ trial. Catheter Cardiovasc Interv 2014;84:236–44
Crossref| PubMed - Zeller T, Langhoff R, Rocha-Singh KJ, et al. Directional atherectomy followed by a paclitaxel-coated balloon to inhibit restenosis and maintain vessel patency: twelve-month results of the DEFINITIVE AR study. Circ Cardiovasc Interv 2017;10:pii: e004848.
Crossref| PubMed - Rastan A, McKinsey JF, Garcia LA, et al. One-year outcomes following directional atherectomy of popliteal artery lesions: subgroup analysis of the prospective, multicenter DEFINITIVE LE trial. J Endovasc Ther 2018;25:100–8
Crossref| PubMed - Cioppa A, Stabile E, Salemme L, et al. Combined use of directional atherectomy and drug-coated balloon for the endovascular treatment of common femoral artery disease: immediate and one-year outcomes. Eurointervention 2017;12:1789–94.
Crossref| PubMed - Schwindt AG, Bennett JG, Crowder WH, et al. Lower extremity revascularization using optical coherence tomography-guided directional atherectomy: final results of the EValuatIon of the PantheriS OptIcal COherence Tomography ImagiNg Atherectomy System for use in the Peripheral Vasculature (VISION) Study. J Endovasc Ther 2017;24:355–66
Crossref| PubMed - Zeller T, Krankenberg H, Steinkamp H, et al. One-year outcome of percutaneous rotational atherectomy with aspiration in infrainguinal peripheral arterial occlusive disease: the multicenter pathway PVD trial. J Endovasc Ther 2009;16:653–62
Crossref| PubMed - Sixt S, Rastan A, Scheinert D, et al. The 1-year clinical impact of rotational aspiration atherectomy of infrainguinal lesions. Angiology 2011;62:645–56
Crossref| PubMed - Mehta M, Zhou Y, Paty PSK, et al. Percutaneous common femoral artery interventions using angioplasty, atherectomy, and stenting. J Vasc Surg 2016;64:369–79
Crossref| PubMed - Gray WA, Garcia LA, Amin A, et al. Jetstream atherectomy system treatment of femoropopliteal arteries: results of the post-market JET registry. Cardiovasc Revasc Med 2018;19:506–11
Crossref| PubMed - Banerjee A, Sarode K, Mohammad A, et al. Safety and effectiveness of the Nav-6 filter in preventing distal embolization during jetstream atherectomy of infrainguinal peripheral artery lesions. J Invasive Cardiol 2016;28:330–3.
PubMed - Shammas NW, Lam R, Mustapha J, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther 2012;19:480–8
Crossref| PubMed - Safian RD, Niazi K, Runyon JP, et al. Orbital atherectomy for infrapopliteal disease: device concept and outcome data for the OASIS trial. Catheter Cardiovasc Interv 2009;73:406–12
Crossref| PubMed - Das T, Mustapha J, Indes J, et al. Technique optimization of orbital atherectomy in calcified peripheral lesions of the lower extremities: the CONFIRM series, a prospective multicenter registry. Catheter Cardiovasc Interv 2014;83:115–22
Crossref| PubMed - Adams GL, Das T, Lee MS, et al. Subanalysis of the CONFIRM registries: acute procedural outcomes in claudicant and critical limb ischemia patients with varying levels of calcification treated for peripheral arterial disease with orbital atherectomy. J Invasive Cardiol 2015;27:516–20.
PubMed - Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360° Trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol 2014;26:355–60.
PubMed - Lee MS, Heikali D, Mustapha J, et al. Acute procedural outcomes of orbital atherectomy for the treatment of common femoral artery disease: Sub-analysis of the CONFIRM Registries. Vasc Med Lond Engl 2017;22:301–6
Crossref| PubMed - Lee MS, Martinsen BJ, Hollowed J, et al. Acute procedural outcomes of orbital atherectomy for the treatment of iliac artery disease: Sub-analysis of the CONFIRM registries. Cardiovasc Revasc Med 2018;19:503–5
Crossref| PubMed - Lee MS, Srivastava PK, Al Yaseen S, et al. Acute procedural outcomes of orbital atherectomy for the treatment of profunda femoris artery disease: subanalysis of the CONFIRM registries. J Invasive Cardiol 2018;30:177–81.
PubMed - Foley TR, Cotter RP, Kokkinidis DG, et al. Mid-term outcomes of orbital atherectomy combined with drug-coated balloon angioplasty for treatment of femoropopliteal disease. Catheter Cardiovasc Interv 2017;89:1078–85
Crossref| PubMed - Davis T, Ramaiah V, Niazi K, et al. Safety and effectiveness of the Phoenix Atherectomy System in lower extremity arteries: early and midterm outcomes from the prospective multicenter EASE study. Vascular 2017;25:563–75
Crossref| PubMed - Radoux JM, Maïza D, Coffin O. Long-term outcome of 121 iliofemoral endarterectomy procedures. Ann Vasc Surg 2001;15:163–70
Crossref| PubMed - Mellière D, Blancas AE, Desgranges P, Becquemin JP. The underestimated advantages of iliofemoral endarterectomy. Ann Vasc Surg 2000;14:343–9
Crossref| PubMed - Kang JL, Patel VI, Conrad MF, et al. Common femoral artery occlusive disease: contemporary results following surgical endarterectomy. J Vasc Surg 2008;48:872–7.e1
Crossref| PubMed - Kechagias A, Ylönen K, Biancari F. Long-term outcome after isolated endarterectomy of the femoral bifurcation. World J Surg 2008;32:51–4
Crossref| PubMed - Nguyen B-N, Amdur RL, Abugideiri M, et al. Postoperative complications after common femoral endarterectomy. J Vasc Surg 2015;61:1489–1494.e1
Crossref| PubMed
