Jason Cottrell, Mitchell Silver
|
Abstract Historically, anticoagulation has been the primary treatment for acute lower extremity deep venous thrombosis (DVT) with or without thrombolysis. Despite large amounts of clinical research data supporting an ‘open vein hypothesis’, which favours early thrombus removal, clinicians have been hesitant to take this option due to a historically high risk of major bleeding and a few notable studies that failed to show any meaningful benefit. The ATTRACT trial failed to show the benefit of using pharmacomechanical catheter-directed thrombolysis (PCDT) for iliofemoral and femoropopliteal DVT but did result in more bleeding. However, the CaVent study before it revealed a significant reduction in post-thrombotic syndrome (PTS) for patients with iliofemoral and femoral DVT after long-term follow-up past 24 months that grew over time. Since these trials, there have been significant advancements in magnetic resonance and CT venography, intravascular ultrasound (IVUS), venous stenting and thrombectomy catheters meaning there is little to no need for adjunct thrombolytics. Results from ongoing research such as CLEAR-DVT reflect the advances in modern technology. Keywords: Deep vein thrombosis, residual venous obstruction, post thrombotic syndrome Disclosure: MJS has recieved consulting fees from Inari Medical, Boston Scientific, Gore Medical and Cook Medical and speaker fees from Bristol Myers Squibb, Pfizer, Astra Zeneca and Portola Pharmaceuticals; he is a stockholder for Contego Medical. JC has no conflicts of interest to declare. Received: 13 August Accepted: 10 June 2022 Published online:24-Nov-2022 Correspondence Details: :Mitchell J Silver, Center for Critical Limb Care and Endovascular Research, OhioHealth Heart and Vascular, Riverside Methodist Hospital, 3705 Olentangy River Road, Columbus, OH 43214, US. E: Mitch.Silver@OhioHealth.com Copyright Statement:
This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly
|
The goals for treatment of acute deep venous thrombosis (DVT) have expanded as the understanding of the pathophysiology behind venous thromboembolism has evolved. There are four generally accepted goals for the treatment of lower extremity DVT. Those goals are to diminish the severity and duration of acute lower extremity symptoms, prevent pulmonary embolism, minimise the risk of recurrent venous thrombosis, and (prevent post-thrombotic syndrome (PTS). One argument against treating all DVT aggressively is the associated risk of major bleeding and common practice relies heavily on the use of thrombolytic agents, anticoagulation medications and procedures requiring endovascular access. Notwithstanding, the most recent multicentre randomised controlled trials (RCTs) regarding acute DVT intervention (ATTRACT, CaVenT, CAVA) all found weak effects from endovascular thrombus removal. Fortunately, the vascular community has witnessed a rapid refinement of endovascular devices and thrombotic therapy that reduce bleeding risk by requiring little to no adjunct thrombolysis. Such a monumental change calls into question the modern-day applicability of conclusions from previous foundational – and at times conflicting – vascular research that weighed the risks of bleeding against the benefits of acute intervention.
By the mechanism of action, conventional anticoagulation works to prevent further propagation of thrombus. Patients with acute DVT who are treated solely with anticoagulation, therefore rely on their innate fibrinolytic pathways to dissolve the clot if vein patency is to be re-established. Such resolution can be slow and, in many cases, the entirety of the residual vein thrombosis (RVT) never completely dissipates.
DVT that is unsuccessfully treated can lead to venous reflux and venous hypertension that builds with time. The resultant venous hypertension then causes increased capillary bed pressures which cause inflammatory oedema and endothelial remodelling. When these physiological changes produce symptoms, PTS is diagnosed. PTS typically manifests as pain, swelling and skin changes of various degrees of severity from mild to severely debilitating. One survey of patients suffering from PTS reported quality of life measurements comparable to those reported by patients with chronic angina, heart failure and many cancers.1 Certain patient characteristics increase the risk of developing PTS (Table 1).
The prevention of PTS has been a focus of recent research, with conflicting evidence regarding its management. In the past, therapy centred around external venous compression; however, one RCT showed that DVT patients who wear elastic compression stockings daily after a proximal DVT showed no improvement in PTS.2 Furthermore, a meta-analysis of 674 reports suggested that the current body of evidence for compression therapy was limited and further studies of compression stockings to prevent PTS were needed.3
The purpose of this review is to outline the evolution in acute DVT management which now encompasses better patient selection, care pathways for earlier endovascular management and catheter thrombectomy technology that is more efficient and often requires minimal or no adjunctive thrombolytic medication.
Patient Characteristics Associated with Increased Risk of Developing Post-thrombotic Syndrome Following Deep Vein Thrombosis
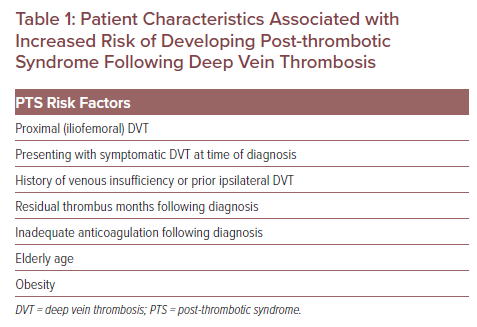
Ultrasound Imaging of a Left External Iliac Vein
Open Vein Hypothesis
It has long been hypothesised that rapid restoration of venous outflow by early thrombus removal can prevent valvular reflux and complications of PTS. The clinical importance of RVT as a determinant for the risk of developing PTS has borne out in clinical research for many years.4 Despite this knowledge, the burden of major bleeding risk has dissuaded many physicians from aggressively treating acute DVT as, until recently, systemic thrombolysis has been the only non-surgical option to achieve ‘open vein’ status.
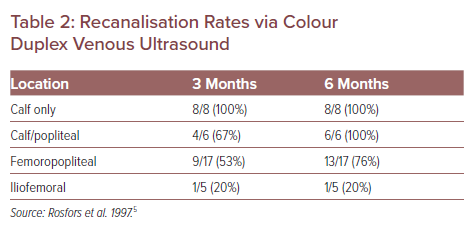
The treatment of lower extremity DVT is typically stratified initially by the location as found on the presenting conventional duplex ultrasound. This stratification is commonly iliofemoral DVT (IF-DVT), femoropopliteal DVT and isolated calf DVT. The notion of ascending versus descending DVT is not part of this stricter anatomical imaging stratification. Skilled technicians can use modern point of care ultrasound to detect RVT with extremely high sensitivity and specificity and essentially no risk to the patient (Figure 1). Duplex ultrasound studies have found that conventional anticoagulation of distal infrapopliteal DVT is efficacious, but when the culprit anatomy is more proximal, such as with IF-DVT, the recanalisation rates on a venous duplex at 6 months are less robust and have higher rates of RVT (Table 2).5 Hypothetically, if patients with IF-DVT received early interventions for thrombus removal and patients with more distal DVT were monitored on anticoagulation alone, RVT would be minimised and fewer patients would suffer moderate-to-severe PTS.
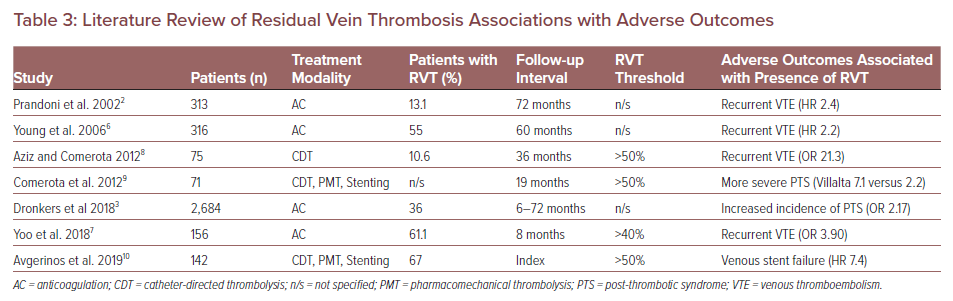
With this hypothesis in mind, a search for literature specifically assessing adverse outcomes associated with patients that had RVT after DVT identified seven peer-reviewed studies, comprising data from more than 3,000 patients. The data from those studies produced a pattern of three clinically relevant outcomes (Table 3). RVT after DVT was repeatedly associated with recurrent venous thromboembolism (VTE) (HR 2.2 and 2.4; OR 3.9 and 21.3).2,6–8 The presence of RVT after DVT was predictive of developing PTS (OR 2.17) and patients with RVT suffered more severe PTS symptoms (mean Villalta PTS severity score 7.1 versus 2.2).3,9,10 One recent study identified RVT as the single most powerful predictor of venous stent failure (HR 7.4).10 Beyond these seven studies, there are two others – CaVenT and ATTRACT – with seemingly contradictory conclusions that should be further analysed side by side.
CaVenT
The CaVenT study randomised 209 patients from 20 hospitals in Norway who presented with a first-time acute DVT that was either femoral or iliofemoral.11 The intention was to treat one arm of patients with catheter-directed thrombolysis (CDT) in addition to standard anticoagulation, treat the other arm with anticoagulation-based treatment alone and follow the clinical outcomes to see if thrombolysis reduced the risk of PTS. At 24 months, patients in the CDT arm experienced an absolute risk reduction for having PTS of 14.4% compared to conventional anticoagulation alone. Long-term follow-up results of the CaVenT trial showed this same absolute risk reduction increase to 28% after 5 years (42.5% versus 70.8%). Collecting such enduring data dropped the already low number needed to treat from seven to four.12 As qualification of PTS for these patients, 83.8% of cases in the CDT arm and 77.8% of the cases in the standard treatment arm were mild (Villalta score 5–9). Differences in quality of life (QOL) indices measured did not reach statistical significance.
Regarding the open vein hypothesis, the CaVenT study found that iliofemoral vein patency after 6 months was more often present in the CDT group and patency after 24 months correlated with residual thrombus burden after completing CDT treatment. Interestingly, regarding the effects on PTS itself, the end-of-procedure venogram result (i.e. the amount of residual thrombus) did not correlate with either 2-year PTS (binary) or with the continuous 2-year Villalta scores. The CaVenT study therefore, does suggest that maintenance of an open vein may best correlate with improved late clinical outcomes rather than early thrombus removal.
ATTRACT
The ATTRACT trial was an RCT designed to evaluate pharmacomechanical catheter-directed thrombolysis (PCDT) as a method for the prevention of PTS in patients with proximal DVT.13 The first arm underwent a PCDT protocol in addition to conventional anticoagulation. The control arm received conventional anticoagulation only. The protocol used three different catheter modalities and all patients in the intervention arm were given catheter-directed tissue plasminogen activator (tPA) as the thrombolytic agent. The primary efficacy outcome was the development of PTS, defined as a score of 5 or greater on the Villalta PTS scale or venous ulceration at any time from the 6-month post-randomisation follow-up visit to the 24-month visit. Over 24 months, investigators were unable to detect any statistically significant relative risk reduction of PTS between the two trial arms. Of the patients, 47% that received PCDT and 48% of non-PCDT patients met the primary outcome (ARR <0.01; RRR 0.02, 95% CI [0.82–1.11]; p=0.56).
The ATTRACT study did find that PCDT reduced PTS severity and improved venous QOL in the subgroup of IF-DVT patients.14 For the IF-DVT subgroup, symptom severity scores were higher (worse), and venous disease-specific QOL scores were lower (worse) in patients with greater post-PCDT thrombus volume over 1 and 24 months, with the difference reaching statistical significance for the 24-month Villalta PTS severity score (p=0.0098).15
The ATTRACT investigators deserve congratulations on executing this important body of work. However, the field of venous intervention has gained considerable experience and improvement in technology since ATTRACT was initiated in 2009. Therefore, the nearly 50% rate of PTS – found not only in the ATTRACT trial but also in VETO, CaVenT, and CAVA – is not satisfactory.11,16,18 Clinicians and industry partners are actively working on procedural and technological advances.
Technological Advancements
There has been an ongoing effort to improve the efficacy of stand-alone CDT in reducing thrombus burden in acute IF-DVT. One such development is ultrasound-assisted catheter-directed thrombolysis (USAT), combining CDT with a catheter system that uses high-frequency, low-power ultrasound.18 According to in vitro studies, ultrasound causes reversible disaggregation of uncrosslinked fibrin fibres, which when combined with ultrasound pressure waves increases the thrombus penetration of thrombolytic drugs.19–21 Unfortunately, the clinical benefit of these USAT devices remains uncertain.
When CDT was compared with USAT for the treatment of patients with acute IF-DVT in the BERNUTIFUL trial, no statistically significant difference in PTS symptoms or thrombus load reduction was found between the two treatment groups.22 In addition, the CAVA trial, a multicentre RCT that compared USAT in addition to standard therapy against standard therapy alone in patients with acute IF-DVT found no benefits to USAT in terms of PTS or QOL at 1-year follow-up.17
Over the past few years, there have been substantial advances in catheter-based thrombectomy devices which allow for more efficient and faster treatment times as well as reducing or even eliminating a need for adjunctive thrombolytic medications. These newer thrombectomy devices are the focus of current studies and will hopefully be found to have a positive impact on reducing RVT which should decrease the incidence of PTS.
In addition, advances in the care pathway of the lower extremity DVT patient have led to more thoughtful pre-procedural planning. Advanced imaging such as MR venography (MRV) or CT venography (CTV) are non-invasive but provide vast amounts of data to contextualise DVTs. Their use prior to intervention can characterise the presence of extrinsic compression, better assess the overall thrombus burden, and identify important concomitant pathologies such as tumours or masses. Having those data before attempting endovascular intervention allows for a more tailored approach which helps drive efficiency and device selection.
The use of intravascular ultrasound (IVUS) to guide venous intervention is an especially important advancement as highlighted by the VIDIO trial which compared the diagnostic efficacy of multiplanar venography combined with IVUS against venography alone for iliofemoral vein obstruction.23 Results demonstrated that the addition of IVUS improved predictive imaging accuracy and guided decision-making for the treatment of iliofemoral venous lesions. In fact, in this series, IVUS changed the treatment plan by the type of therapy in 60 of 100 patients and determined the need for venous stenting in 50 of 100 patients. The VIDIO trial concluded that without IVUS, iliofemoral vein occlusive disease would have been undertreated in the majority of patients studied.
Recanalisation Rates via Colour Duplex Venous Ultrasound
There is a common thread from the CAVA and CaVenT studies and the IF-DVT patients of the ATTRACT trial, which inspires hope for a future in which it is possible to predict which patients will benefit most from early thrombus removal and the patients at greatest risk of developing PTS could receive the most advanced therapies giving them the greatest chance of lasting vein patency without PTS symptoms.
CLEAR-DVT
In an effort to build on the findings from studies such as CaVenT and ATTRACT, the CLEAR-DVT study (NCT03901872) was designed to demonstrate that contemporary venous intervention in the right patient population would result in an open vein and dramatically decrease PTS in patients with acute IF-DVT.
The CLEAR-DVT investigators started with a small prospective pilot study with no control group as a proof of principle study in patients with acute IF-DVT. It was funded by Boston Scientific and sponsored by Guy’s and St Thomas’ Hospital in London, England. The results gathered by the CLEAR-DVT cohort pilot study would then be used to power and plan a formal RCT of modern venous intervention for patients with acute IF-DVT.
The phase 1 cohort of CLEAR-DVT was a prospective pilot study with no control group comprising 35 patients (39% male, 61% female; average age 48 years, range 22–73 years) with acute IF-DVT and symptom onset of 14 days or less. The average Villalta score prior to intervention was 10 (range 4–19). All patients underwent pharmacomechanical thrombolysis (PMT) in the form of the 8F Zelante AngioJet catheter using a dose of thrombolytic that was standard of care for each investigational site. The use of IVUS was mandated in all patients and venous stenting was performed for any patients with >50% cross-sectional area reduction. All patients received standardised post-procedural anticoagulation. Follow up of the first 23 patients at 6 months post procedure demonstrated an average Villalta score of 2; 96% of patients had no PTS at all (4% had mild PTS and none had a Villalta score >5). All patients will be followed for 1 year for the Villalta score as well as a 6-minute walk test, venous clinical severity score and QOL scores.
The importance of early complete thrombus removal was suggested by the findings in the clear-DVT pilot cohort that demonstrated a dramatic reduction in PTS in patients with acute IF-DVT undergoing IVUS-guided contemporary venous intervention (Table 4). Certainly, these findings need to be validated in a formal RCT of contemporary venous intervention using lytic- and non-lytic based therapies against best medical therapy.
Literature Review of Residual Vein Thrombosis Associations with Adverse Outcomes
Conclusion
The field of venous intervention is dynamic. We now have mechanical thrombectomy catheters that remove thrombus efficiently without the need for adjunctive thrombolytics. Such technology will certainly make venous intervention safer with less bleeding complications. These devices will likely be effective at reducing RVT as they are known to be able to clear all or most of the thrombus acutely in a single setting. Additionally, dedicated venous stents that are engineered and purpose-built for vein anatomy will be an incredibly positive addition to our treatment options.
It is encouraging that the field of venous intervention has made major advances in selecting the right patient population to treat acute lower extremity DVT, how to perform a high-quality venous intervention with IVUS guidance, and now has catheter thrombectomy systems that reduce and even eliminate the need for thrombolysis in the majority of acute IF-DVT patients. Altogether these innovations will hopefully provide momentum to continue evolving procedures that are even safer and more effective. In so doing, the treatable patient population will continue to expand by including more and more patients with contraindications to thrombolysis.
Patient Characteristics and Preliminary Data of CLEAR-DVT
In conclusion, complete early thrombus resolution in patients with acute IF-DVT will reduce RVT and hopefully translate to less PTS in high-risk patient populations. Treatment pathways with this specific objective will positively impact the prognosis and quality of life of patients with acute lower extremity DVT.
References
-
- Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost 2008;6:1105–12.
Crossref| PubMed - Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
Crossref| PubMed - Dronkers CEA, Mol GC, Maraziti G, et al. Predicting post-thrombotic syndrome with ultrasonographic follow-up after deep vein thrombosis: a systematic review and meta-analysis. Thromb Haemost 2018;118:1428–38.
Crossref| PubMed - Singh H, Masuda EM. Comparing short-term outcomes of femoral-popliteal and iliofemoral deep venous thrombosis: early lysis and development of reflux. Ann Vasc Surg 2005;19:74–9.
Crossref| PubMed - Rosfors S, Eriksson M, Leijd B, Nordström E. A prospective follow-up study of acute deep venous thrombosis using colour duplex ultrasound, phlebography and venous occlusion plethysmography. Int Angiol 1997;16:39–44.
PubMed - Young L, Ockelford P, Milne D, et al. Post-treatment residual thrombus increases the risk of recurrent deep vein thrombosis and mortality. J Thromb Haemost 2006;4:1919–24.
Crossref| PubMed - Yoo T, Aggarwal R, Wang TF, et al. Presence and degree of residual venous obstruction on serial duplex imaging is associated with increased risk of recurrence and progression of infrainguinal lower extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord 2018;6:575–583.e1.
Crossref| PubMed - Aziz F, Comerota AJ. Quantity of residual thrombus after successful catheter-directed thrombolysis for iliofemoral deep venous thrombosis correlates with recurrence. Eur J Vasc Endovasc Surg 2012;44:210–3.
Crossref| PubMed - Comerota AJ, Grewal N, Martinez JT, et al. Post-thrombotic morbidity correlates with residual thrombus following catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg 2012;55:768–73.
Crossref| PubMed - Avgerinos ED, Saadeddin Z, Abou Ali AN, et al. Outcomes and predictors of failure of iliac vein stenting after catheter-directed thrombolysis for acute iliofemoral thrombosis. J Vasc Surg Venous Lymphat Disord 2019;7:153–61.
Crossref| PubMed - Enden T, Haig Y, Kløw NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379:31–8.
Crossref| PubMed - Haig Y, Enden T, Grøtta O, et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol 2016;3:e–71.
Crossref| PubMed - Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med 2017;377:2240–52.
Crossref| PubMed - Comerota AJ, Kearon C, Gu CS, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation 2019;139:1162–73.
Crossref| PubMed - Weinberg I, Vedantham S, Salter A, et al. Relationships between the use of pharmacomechanical catheter-directed thrombolysis, sonographic findings, and clinical outcomes in patients with acute proximal DVT: results from the ATTRACT multicenter randomized trial. Vasc Med 2019;24:442–51.
Crossref| PubMed - Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149:698–707.
Crossref| PubMed - Notten P, Ten Cate-Hoek AJ, Arnoldussen CWKP, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol 2020;7:e40–9.
Crossref| PubMed - Engelberger RP, Kucher N. Ultrasound-assisted thrombolysis for acute pulmonary embolism: a systematic review. Eur Heart J 2014;35:758–64.
Crossref| PubMed - Blinc A, Francis CW, Trudnowski JL, Carstensen EL. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood 1993;81:2636–43.
Crossref| PubMed - Francis CW, Blinc A, Lee S, Cox C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol 1995;21:419–24.
Crossref| PubMed - Braaten JV, Goss RA, Francis CW. Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost 1997;78:1063–8.
Crossref| PubMed - Engelberger RP, Spirk D, Willenberg T, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv 2015;8:1–9.
Crossref| PubMed - Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord 2017;5:678–87.
Crossref| PubMed
- Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost 2008;6:1105–12.
