Raghu Kolluri, William A Gray, Ehrin Armstrong, Brian C Fowler
|
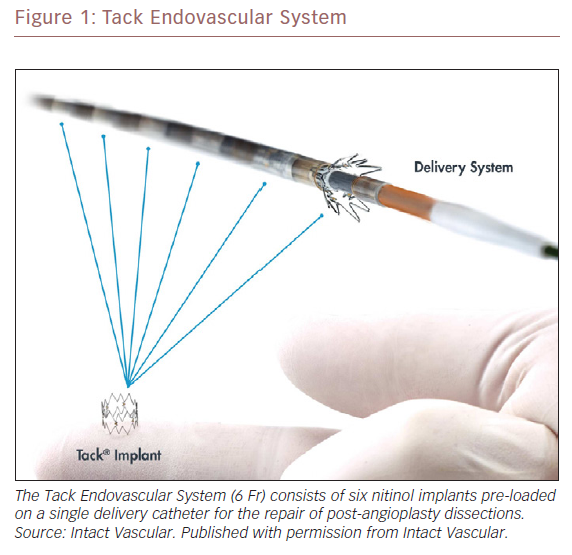
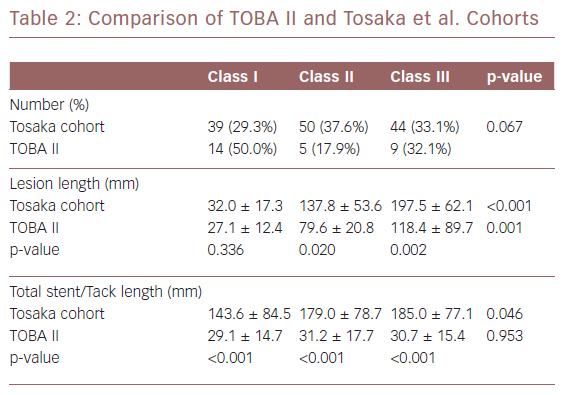
Abstract In-stent restenosis is complex, difficult to treat and has led to a ‘leave less metal behind’ approach to femoropopliteal intervention. Postangioplasty dissection often requires scaffolding to maintain patency. The Tack Endovascular System provides minimal-metal dissection repair that preserves future treatment options. Tack implants are designed to minimise the inflammation and neointimal hyperplasia that lead to in-stent restenosis. An independent angiographic core laboratory evaluated the restenosis patterns in clinically driven target lesion revascularisation (CD-TLR) during the 12 months following the index procedure in the Tack Optimized Balloon Angioplasty (TOBA) II study and compared these results to those published for nitinol stent implantation. Of the 213 patients in TOBA II, 31 (14.6%) required a CD-TLR. Of these, 28 had angiograms that were evaluated by the core laboratory and 45.2%, 16.1%, and 29% were graded as Tosaka class I, II and III, respectively. There were no significant differences (p>0.05) in lesion length, degree of calcification or dissection class between the three groups. Tack restenotic lesion classification and analysis show a prevalence of both class I and shorter lesions relative to in-stent restenosis, which may be beneficial to long-term patient outcomes.
Disclosure: This study was funded by Intact Vascular. RK and BF are employees of Syntropic Core Laboratory. WG is the National Principal Investigator for TOBA II. EA is an investigator for TOBA II.
Received: Accepted: Published online:
Correspondence Details: Raghu Kolluri, 3535 Olentangy River Rd, Suite 514, Columbus, OH 43214, US. E: Raghu.Kolluri@ohiohealth.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
|
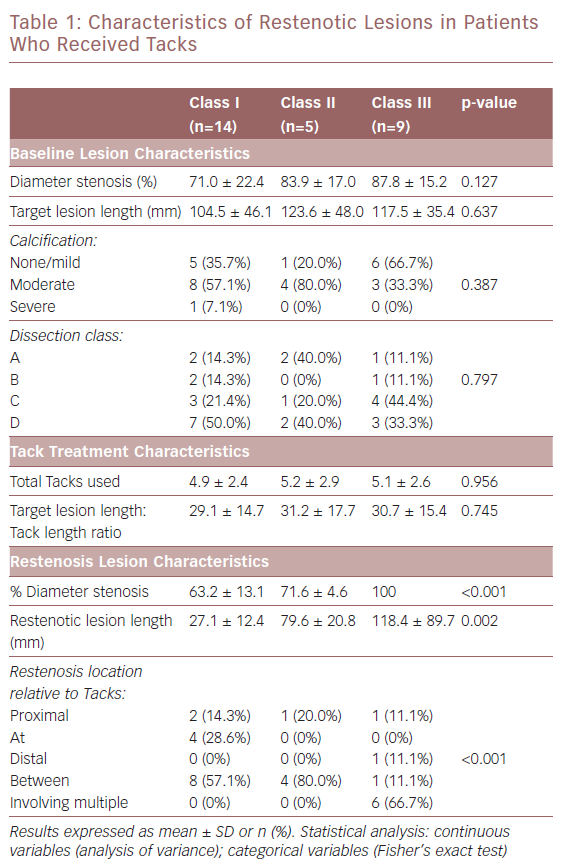
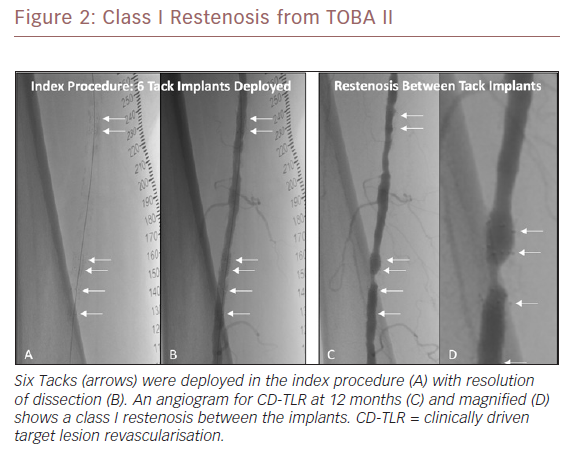
The restenotic lesions were evaluated for per cent diameter occlusion and lesion length. They were classified using the methodology of Tosaka et al. as follows: class I – focal (<50 mm in length) lesions located within the stent body, at the stent edge, or a combination of both; class II – diffuse (>50 mm in length) including both stent body and stent edge lesions; and class III – total occlusion. As noted, the Tosaka classification system was developed to describe lesions in full-length stents. Unlike stents, Tacks, by design, do not cover the full length of treated lesions and multiple Tacks can be used to treat dissections. Due to this unique feature, the core lab also provided an analysis of the location of target lesion restenosis relative to Tack location(s) using the following qualitative analysis:
- proximal – lesion located proximal to an area that was Tacked;
- at – lesion located within a Tack;
- distal – lesion located distal to a Tacked area;
- between – lesion located between Tacks; and
- involving multiple – lesions located at multiple Tacks.
Characteristics of Restenotic Lesions in Patients Who Received Tacks
- Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. Crossref| PubMed
- Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol 2016;19:91–5. Crossref| PubMed
- Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26. Crossref| PubMed
- Rooke TW, Hirsch AT, Misra S, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:1555–70. Crossref| PubMed
- Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Eur Heart J 2018;39:763–816. Crossref| PubMed
- Werk M, Albrecht T, Meyer DR, et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: Evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv 2012;5:831–40. Crossref| PubMed
- Tepe G, Zeller T, Schnorr B, et al. High-grade , non-flow-limiting dissections do not negatively impact long-term outcome after paclitaxel-coated balloon angioplasty: an additional analysis from the THUNDER study. J Endovasc Ther 2013;20:792–800. Crossref| PubMed
- Fujihara M, Takahara M, Sasaki S, et al. Angiographic dissection patterns and patency outcomes after balloon angioplasty for superficial femoral artery disease. J Endovasc Ther 2017;24:367–75. Crossref| PubMed
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007;33:S1–75. Crossref| PubMed
- Rocha-Singh KJ, Jaff MR, Crabtree TR, et al. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv 2007;69:910–9; PMID: 17377972. PubMed
- Werk M, Langner S, Reinkensmeier B, et al. Inhibition of restenosis in femoropopliteal arteries. Paclitaxel-coated versus uncoated balloon: Femoral paclitaxel randomized pilot trial. Circulation 2008;118:1358–65. Crossref| PubMed
- Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008;358:689–99. Crossref| PubMed
- Schillinger M, Sabeti S, Loewe C, et al. Balloon Angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006;354:1879–88. Crossref| PubMed
- Krankenberg H, Schlüter M, Steinkamp HJ, et al. Transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the Femoral Artery Stenting Trial (FAST). Circulation 2007;116:285–92. Crossref| PubMed
- Bosiers M, Torsello G, Gissler HM, et al. Nitinol stent implantation in long superficial femoral artery lesions: 12-month results of the DURABILITY I study. J Endovasc Ther 2009;16:261–9. Crossref| PubMed
- Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: Twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv 2010;3:267–276. Crossref| PubMed
- Gray W. TOBA II 12-month results: Tack optimized balloon angioplasty. Presented at: VIVA 2018, Las Vegas, NV, 5–8 November 2018.
- Garcia LA, Rocha-Singh KJ, Krishnan P, et al. Angiographic classification of patterns of restenosis following femoropopliteal artery intervention: a proposed scoring system. Catheter Cardiovasc Interv 2017;90:639–646. Crossref| PubMed
- Tosaka A, Soga Y, Iida O, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol 2012;59:16–23. Crossref| PubMed
- Coronary artery angiographic changes after PTCA: In: Manual of Operations NHLBI PTCA Registry. Bethesda, MD: National Heart, Lung, and Blood Institute, 1985;6–9.
- Ballyk PD. Intramural stress increases exponentially with stent diameter: a stress threshold for neointimal hyperplasia. J Vasc Interv Radiol 2006;17:1139–1145. Crossref| PubMed
- Freeman JW, Snowhill PB, Nosher JL. A link between stent radial forces and vascular wall remodeling: the discovery of an optimal stent radial force for minimal vessel restenosis. Connect Tissue Res 2010;51:314–26. Crossref| PubMed
- Zhao HQ, Nikanorov A, Virmani R, et al. Late stent expansion and neointimal proliferation of oversized nitinol stents in peripheral arteries. Cardiovasc Intervent Radiol 2009;32:720–6. Crossref| PubMed
- Schneider PA, Giasolli R, Ebner A, et al. Early experimental and clinical experience with a focal implant for lower extremity post-angioplasty dissection. JACC Cardiovasc Interv 2015;8:347–54. Crossref| PubMed
- Armstrong EJ, Singh S, Singh GD, et al. Angiographic characteristics of femoropopliteal in-stent restenosis: Association with long-term outcomes after endovascular intervention. Catheter Cardiovasc Interv 2013;82:1168–74. Crossref| PubMed
