Ceazón T Edwards, Peter A Schneider, Cindy Huynh
|
Abstract The role of paclitaxel in the treatment of femoropopliteal peripheral arterial disease is currently ambiguous. A summary-level meta-analysis of randomised trials published in 2018 demonstrated that paclitaxel-coated devices were associated with an increased all-cause mortality in those who underwent treatment at 2 years and 5 years. Further evaluation has been undertaken to establish whether there is a specific dose response, mechanism or reproducible signal. At this time, there has been no confirmation of dose response, as was initially asserted by the summary-level meta-analysis. No mechanism of harm has been identified. Although an association with increased mortality has been confirmed by patient-level meta-analysis, the strength of the signal has been inconsistent. The information suggests there is only an association between paclitaxel-coated devices and increased all-cause mortality, not causation. The authors encourage additional studies designed to follow long-term results after treatment with paclitaxel-coated devices, using real patient data, before a conclusion can be made. Keywords: Paclitaxel, femoropopliteal, femoral popliteal, drug-eluting stents, percutaneous transluminal angioplasty, mortality, peripheral arterial disease Disclosure: PAS has served as a consultant for Surmodics, Silk Road Medical, Medtronic, CSI, Profusa, Intact Vascular, Cagent, Illuminate, Intervene, Limflow, Devoro, PQ Bypass, Boston Scientific and Philips. All other authors have no conflicts of interest to declare. Received: Accepted:Published online: Correspondence Details: Ceazón T Edwards, Division of Vascular and Endovascular Surgery, University of California San Francisco, 400 Parnassus Ave, A-581, San Francisco, CA 94143, US. E: ceazon.edwards@ucsf.edu Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
|
The treatment of peripheral artery disease (PAD), as related to lifestyle-limiting claudication and chronic limb-threatening ischaemia, has changed drastically over the past 20 years with the advent of percutaneous intervention as an alternative to open surgery. More recently, the use of drug-coated devices (more specifically, paclitaxel-coated devices) has been shown to reduce the rate of restenosis, reintervention, the subsequent need for target limb revascularisation and lower extremity amputation rate in comparison with non-drug-coated devices.1 Paclitaxel is a common anti-neoplastic, anti-microtubular agent originally approved as a first-line treatment in several solid-organ carcinomas in the 1960s.2 Its biochemical and pharmacological properties are involved in the inhibition and prevention of cellular division.
Similarly, as paclitaxel has also been used in drug-coated coronary devices in contemporary medicine, its properties have also been extrapolated in the treatment of PAD within the femoropopliteal segment due to the promise of anti-stenotic properties. First-generation paclitaxel-coated coronary devices in the early 2000s were associated with late stent thrombosis and have since been falling out of favour.
In 2014, a meta-analysis of 76 different randomised controlled trials demonstrated that sirolimus-coated devices reduced both short- and long-term risks of target lesion revascularisation (TLR), restenosis, major adverse cardiac events and overall risk of MI in comparison with their paclitaxel counterparts.2,3 Although the REALITY trial and other randomised studies showed the superiority of sirolimus over paclitaxel-coated devices in the treatment of coronary artery disease, the use of paclitaxel in the treatment of femoropopliteal PAD is promising.4,5
Although the potential cytotoxicity of paclitaxel in relation to systemic chemotherapy regimens has been well-delineated, additional concerns about its role in the treatment of peripheral arterial disease stem from a potential correlation to increased all-cause mortality, as was suggested in a 2018 study by Katsanos et al.6 More recently, the same group published a meta-analysis of randomised controlled trials involving the use of paclitaxel-coated balloons in the treatment of infrapopliteal peripheral arterial disease. That study emphasised a decreased amputation-free survival in those who underwent drug-coated balloon (DCB) angioplasty with high-dose devices (3.0–3.5 µg/mm2) in comparison with conventional balloon angioplasty, suggesting a cytotoxic dose-dependent harm signal.7
Conversely, the results of a 2019 meta-analysis by Schneider et al. did not show a significant difference in the 5-year mortality rates between those patients treated with percutaneous transluminal angioplasty (PTA) and paclitaxel DCB.8 In addition, of those patients treated with paclitaxel DCB, there was no dose-related increased mortality risk, and of those risk factors found to be predictors of increased mortality (which was largely related to the underlying disease process itself), exposure to paclitaxel was not found to be one of them.
Similarly, Secemsky et al. performed a multicentre retrospective cohort study using Medicare and Medicaid real-world data comparing drug-coated devices with standard non-drug-coated devices in the treatment of femoropopliteal PAD. Multivariate analysis suggested there was no difference in all-cause mortality between the two groups, which was consistent across patients with and without critical limb ischaemia and irrespective of the type of drug-coated device used (DCB or drug-eluting stent).9
More recently, Behrendt et al. also used real-world data to suggest increased long-term survival, amputation-free survival and decreased incidence of major cardiovascular events in those treated with drug-coated devices.10
It is easy to understand how the use of drug-coated devices in the treatment of femoropopliteal disease has become a topic of controversy, given the conflicting data. With this, the purpose of this review is to discuss the benefits and risks of exposure to paclitaxel, and to further understand the relationship between drug dose, exposure and its relationship to mortality.
Paclitaxel Mechanism of Action
Paclitaxel is a chemotherapeutic agent at high concentrations, acting to inhibit cell division and promote microtubule assembly, subsequently preventing microtubule breakdown and arresting the cell cycle in the G2/M phase.11 Paclitaxel also inhibits secretion of the extracellular matrix and proliferation of vascular smooth muscle cells and fibroblasts, as well as preventing migration of smooth muscle cells, fibroblasts and white blood cells.12 At lower concentrations, paclitaxel has been found to reduce restenosis, and continues to have a long-term inhibitory effect, even after short exposure time.13
Paclitaxel is lipophilic, allowing for rapid uptake into tissues at high concentrations within the intima of the artery, and low concentrations of the drug in the plasma. The delivery of the hydrophobic, lipophilic paclitaxel as the active component of drug-coated devices is facilitated by a hydrophobic delivery molecule, which allows for its absorption within the arterial wall over time.
When investigated in PAD after treatment with paclitaxel-coated balloon (PCB), paclitaxel was undetectable in plasma by 24 hours with use of up to three balloons and no paclitaxel-related events occurred.14 The known side-effects of paclitaxel at chemotherapeutic concentrations include neutropenia, neuropathy, hypersensitivity and cardiovascular effects, such as hypotension, hypertension, bradycardia, myalgia, myelotoxicity, anaphylaxis and nausea. In clinical trials, the mean total treatment doses delivered by PCB and paclitaxel-eluting stent (PES) was from 1 mg or less up to 20 mg, depending on lesion size, number of lesions treated and type; in comparison, average doses of paclitaxel for a single chemotherapeutic treatment are approximately 230–300 mg, and a total dose of up to 1,200 mg for multiple treatments.8
The SNAPIST I trial examined paclitaxel administration along with bare-metal stent (BMS) placement for the prevention of restenosis at doses of 10, 30, 70 and 100 mg/m2. Systemic side-effects, including moderate neutropenia, sensory neuropathy and alopecia, were noted with doses of 70 and 100 mg/m2, much higher than those delivered with PCB or PES; however, there has been no supported mechanism of possible paclitaxel-mediated increase in mortality.15
Paclitaxel has been used in the treatment of PAD with both balloon angioplasty and stent placement in the femoropopliteal location. The advantages of balloon angioplasty include avoidance of permanent prosthesis placement, whereas stent placement may be challenging in locations with high mechanical force, as they must withstand compression, flexion leading to deformation, fracture and in-stent restenosis.
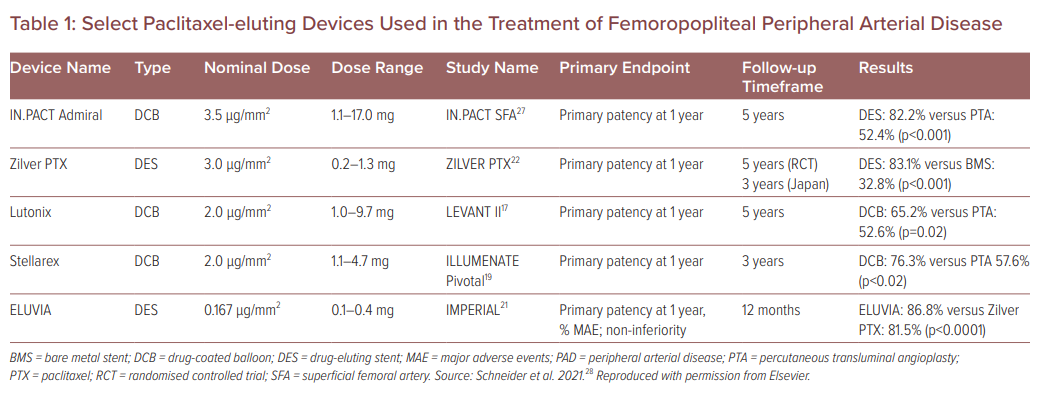
There are currently five Food and Drug Administration (FDA)-approved paclitaxel-coated devices: three PCB and two PESs. The three PCBs include the Lutonix 035 DCB PTA catheter (BD/Bard), IN.PACT Admiral (Medtronic Vascular) and Stellarex (Philips). Several randomised clinical trials reported the benefit of PCB relative to traditional plain PTA. Tepe et al. compared the outcomes of 48 patients treated with PCB and 54 patients treated with plain PTA for superficial femoral and popliteal artery lesions, demonstrating a significant reduction in late lumen loss (0.4 ± 1.2 mm versus 1.7 ± 1.8 mm, p<0.001) and target lesion revascularisation (TLR; 4% versus 29%, p<0.001) at 6 months, and at 24 months.16 Rosenfield et al. found in a single-blinded, randomised study of 475 patients that Lutonix PCB had increased primary patency at 12 months compared with non-drug-coated PTA in femoropopliteal artery disease.17 Although these studies focused on focal lesions less than 10 cm, a post-hoc analysis in the IN.PACT 5-year analysis favoured PCB over PTA in longer lesions, total occlusions and in-stent restenosis.8
Ott et al. compared PCBs with PTA in 70 patients with symptomatic in-stent restenosis of the superficial femoral artery, with a mean lesion length of 13.9 ± 6.7 cm, and found significantly reduced rates of diameter stenosis (44 ± 33% versus 65 ± 33%, p=0.01) and binary restenosis when examined at 6–8 months, as well as reduced rates of TLR at 24 months.18 With this, DCBs appear to be effective and durable interventions superior to PTA for focal lesions at 5 years.
In the ILLUMENATE Pivotal study (Stellarex DCB), the primary patency for DCB was 76.3% compared with 57.6% for PTA (p=0.003) when examined at 12 months, with TLR significantly decreased in the DCB group compared with the PTA group (7.9% versus 16.8%, respectively; p=0.023).19
More recently, in January 2019, Gray et al. performed a meta-analysis with real patient data to determine the safety of the Stellarex DCB in the treatment of femoropopliteal PAD, which revealed no difference in the all-cause mortality in those treated with DCB versus PTA over a 3-year timeframe (1.8 ± 0.7% versus 1.3 ± 0.9% at 1 year, 6.5 ± 1.2% versus 5.9 ± 1.9% at 2 years and 9.3 ± 1.5% versus 9.9 ± 2.4% at 3 years, p=0.86; Kaplan–Meier estimates).20
The two FDA-approved PESs include Eluvia (Boston Scientific) and Zilver PTX (Cook Medical). The Eluvia stent has a polymer coating designed to deliver paclitaxel over a longer period of time than the Zilver PTX, as well as low drug–dose density. The IMPERIAL trial compared the Eluvia PES with the Zilver PTX PES. At 1 year, the Eluvia stent demonstrated non-inferiority to Zilver PTX. Primary patency rates were 86.8% in patients receiving the Eluvia and 81.5% in patients receiving the Zilver PTX (p<0.0001).21
Dake et al. randomised 474 patients in a prospective, multinational trial to PES or PTA. Patients who had initial PTA failure underwent randomisation to PES or BMS. PES was associated with higher 2-year event-free survival (86.6% versus 77.9%, p=0.02) and primary patency (74.8% versus 26.5%, p<0.01) compared with PTA, and also superior 2-year primary patency relative to the BMS group (83.4% versus 64.1%, p<0.01).22 At 5 years, there were higher patency rates (66.4% versus 43.4%, p<0.01) and greater freedom from TLR in patients receiving PES compared with PTA (83.1% versus 67.6%, p<0.01). In addition, at 5 years there were higher primary patency rates of PES to the BMS group (72.4% versus 53%, p=0.03) and freedom from TLR (84.9% versus 71.6%, p=0.06).
Select Paclitaxel-eluting Devices Used in the Treatment of Femoropopliteal Peripheral Arterial Disease
Is the Mortality Increase Associated with Paclitaxel a Causal Relationship or an Association?
Given what we know about the pharmacological properties of paclitaxel and its use in paclitaxel-coated devices, it appears unclear whether increased mortality is simply an association or an actual causation. The 2018 meta-analysis by Katsanos et al. initially reported an increased risk of mortality in those patients who underwent treatment with paclitaxel-coated devices for femoropopliteal disease at 2 and 5 years using meta-regression analysis; however, the methodology behind the dose–mortality calculations used is fundamentally flawed.6
Holden et al. stressed that for a drug to be associated with an adverse event, it must be dose-related, be a consistent result among different patient populations, associated with a certain timeframe and there must be a ‘biological gradient’, as implied by the Bradford Hill criteria.23 In addition, it requires an associated clustering of deaths and/or adverse events, and a reproducible and predictable danger signal, which would insinuate a causal relationship. In the initial article, the proposed biological gradient was the paclitaxel dose-response.
As was described with the comparison among the 4,432 patients in 28 randomised controlled trials, there was no significant difference in all-cause mortality between the paclitaxel arms and the control arm (2.3% crude risk of death in both arms) after 1 year. At 2 years, the all-cause mortality in the paclitaxel group was 7.2% versus 3.8% in the control group, and at 5 years, long-term analysis of all-cause mortality was 14.7% in the paclitaxel group versus 8.1% in the control group.6 Since this claim, there have been several other patient-level randomised trials that have tried to reproduce, but have failed to prove a dose–mortality relationship between paclitaxel dose and exposure over time.
In several of the preclinical models, it was shown that the paclitaxel concentration within the tissues decreases over a 6-month timeframe to nearly undetectable levels. In addition, it would be expected that after initial drug exposure, the longer the patients are prospectively followed, the greater likelihood of there being mortality events in a direct causal relationship.
The aforementioned Katsanos study assumed that there is a continuous linear relationship over time between drug exposure and the severity of the adverse effect; however, several of the named studies used in the meta-analysis were small and not developed with the intent of long-term follow-up. Instead, these studies focused on 1-year patency, which is not enough time to assess long-term results. Not only were the majority of the follow-up intervals short, many of the pivotal randomised controlled trials had a high percentage of patients that were withdrawn from the study or lost to follow-up.
With this information, and in an attempt to reproduce the findings in the initial Katsanos study, Ducasse et al. performed a systematic review and meta-analysis of DCB versus PTA for de novo femoropopliteal lesions, which included 13 different randomised controlled trials that did not show any significant difference in all-cause mortality between the two groups at 1, 2, 4 and 5 years after treatment.24 At 3 years, however, the study did show an increase in mortality, but these deaths were not adjudicated as device or procedure related. Similar to the Katsanos study, the deaths did not include patient-level data, given the endpoints were not highly powered or designed for long-term follow-up, as the design was to focus on primary patency at 6 and 12 months.
More recently, the FDA released an updated report that focused on the long-term mortality results from three pre-market randomised, controlled trials involving the use of paclitaxel-eluting devices in the treatment of femoropopliteal disease: IN.PACT SFA I and II (Medtronic), Cook Zilver PTX and LEVANT 2 (BD/Bard).25 This update also reported an increase in the all-cause mortality in those patients who underwent treatment with paclitaxel-coated devices in comparison with the control group; however, the major flaw is that upon further review, there was no standardised calculation used among the three trials to calculate all-cause mortality, which is subject to bias (e.g. the intention to treat used by BD/Bard in the LEVENT 2 trial used a calculation that excluded pre-existing conditions), and significantly altered the way in which the data were presented and interpreted. As Holden et al. reported using a standardised proportion method for calculating 5-year mortality in these three groups, only the Zilver PTX DES was shown to have a statistically significant difference between the two arms (BMS or PTA) with all-cause mortality at 28.1% (p=0.008), which had the smallest amount of paclitaxel delivery (1.1 mg; Table 1). With these standardised results, a dose–response relationship was not able to be identified, which may imply an association and not a causation.25
With regard to the IN.PACT SFA I (US), II (Europe) and Japan trials, it is important to note that follow-up visit attendance was lower in the DCB arm across all three locations at all time points, which again suggests bias, as patients in the PTA arm may have received more medical care and treatment than those patients in the DCB arm.
In addition, the Zilver PTX, LEVANT 2 and IN.PACT SFA studies had anywhere from 20% to 86% of patients initially enrolled in the study lost to follow-up, to which the FDA responded by requesting the industry partners to resubmit updated follow-up patient numbers for their analysis. Updated patient-level meta-analysis from the Zilver PTX randomised controlled trial and Zilver PTX and B<S Japan post-market studies did not show an increase in long-term all-cause mortality.26 In the clinical trials that are still underway, it will be interesting to see how the COVID-19 pandemic affects data acquisition and patient follow-up.
Conclusion
Early results of the use of paclitaxel-coated devices in the treatment of femoropopliteal PAD are promising. A full and comprehensive study focused on the long-term risks and benefits of paclitaxel using real patient data remains to be ascertained. Without a consistent dose response, underlying pharmacokinetic mechanism and reproducible harm, it is difficult to infer that paclitaxel-coated devices are the cause of increased all-cause mortality. In addition, data amongst randomised controlled trials are not consistent between geographical areas, and the treatment practice of the DCB and PTA arms in each study appear to have been subject to treatment bias, which may account for the inconsistences observed. Again, with the information we currently have, there appears to be only an association between paclitaxel-coated devices and increased all-cause mortality, not a causation.
Before we completely eliminate this promising treatment of recalcitrant peripheral arterial lesions in the lower extremity, we encourage additional studies specifically designed to follow long-term results, and for each clinician to weigh the risks and benefits with regard to each patient, and decide individually if this treatment will allow for the desired outcome; however, more information must be available to make a truly informed decision.
Clinical Perspective
- Paclitaxel is a well-established, potent, antineoplastic agent that has been used in the treatment of femoropopliteal peripheral arterial disease due to its anti-stenotic properties.
- A summary-level meta-analysis demonstrated an association between paclitaxel and increased all-cause mortality.
- An association with increased mortality has been confirmed with patient-level meta-analysis; however, the strength of the signal has been highly variable and no specific mechanism of harm has been identified.
- There exists only an association between paclitaxel-coated devices and increased all-cause mortality, not a causation, which warrants further investigation.
References
- Katsanos K, Spiliopoulos S, Paraskevopoulos I, et al. Systematic review and meta-analysis of randomized controlled trials of paclitaxel-coated balloon angioplasty in the femoropopliteal arteries: role of paclitaxel dose and bioavailability. J Endovasc Ther 2016;23:356–70.
Crossref| PubMed - Mills JL, Conte MS, Murad MH. Critical review and evidence implications of paclitaxel drug-eluting balloons and stents in peripheral artery disease. J Vasc Surg 2019;70:3–7.
Crossref| PubMed - Zhang X, Xie J, Li G, et al. Head-to-head comparison of sirolimus-eluting stents versus paclitaxel-eluting stents in patients undergoing percutaneous coronary intervention: a meta-analysis of 76 studies. PLoS One 2014;9:e97934.
Crossref| PubMed - Morice MC, Colombo A, Meier B, et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA 2006;295:895–904.
Crossref| PubMed - Windecker S, Remondino A, Eberli FR, et al. Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N Engl J Med 2005;353:653–62.
Crossref| PubMed - Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7:e011245.
Crossref| PubMed - Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death and amputation with use of paclitaxel-coated balloons in the infrapopliteal arteries for treatment of critical limb ischemia: a systematic review and meta-analysis of randomized controlled trials. J Vasc Interv Radiol 2020;31:202–12.
Crossref| PubMed - Schneider PA, Laird JR, Doros G, et al. Mortality not correlated with paclitaxel exposure: an independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol 2019;73:2550–63.
Crossref| PubMed - Secemsky EA, Kundi H, Weinberg I, et al. Association of survival with femoropopliteal artery revascularization with drug-coated devices. JAMA Cardiol 2019;4:332–40.
Crossref| PubMed - Behrendt CA, Sedrakyan A, Peters F, et al. Editor’s choice – Long term survival after femoropopliteal artery revascularisation with paclitaxel coated devices: a propensity score matched cohort analysis. Eur J Vasc Endovasc Surg 2020;59:587–96.
Crossref| PubMed - Horwitz SB. Mechanism of action of taxol. Trends Pharmacol Sci 1992;13:134–6.
Crossref| PubMed - Ng VG, Mena C, Pietras C, et al. Local delivery of paclitaxel in the treatment of peripheral arterial disease. Eur J Clin Invest 2015;45:333–45.
Crossref| PubMed - Axel DI, Kunert W, Göggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997;96:636–45.
Crossref| PubMed - Freyhardt P, Zeller T, Kröncke TJ, et al. Plasma levels following application of paclitaxel-coated balloon catheters in patients with stenotic or occluded femoropopliteal arteries. Rofo 2011;183:448–55.
Crossref| PubMed - Margolis J, McDonald J, Heuser R, et al. Systemic nanoparticle paclitaxel (nab-paclitaxel) for in-stent restenosis I (SNAPIST-I): a first-in-human safety and dose-finding study. Clin Cardiol 2007;30:165–70.
Crossref| PubMed - Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008;358:689–99.
Crossref| PubMed - Rosenfield K, Jaff MR, White CJ, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med 2015;373:145–53.
Crossref| PubMed - Ott I, Cassese S, Groha P, et al. ISAR-PEBIS (Paclitaxel-Eluting Balloon Versus Conventional Balloon Angioplasty for In-Stent Restenosis of Superficial Femoral Artery): a randomized trial. J Am Heart Assoc 2017;6:e006321.
Crossref| PubMed - Krishnan P, Faries P, Niazi K, et al. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE Pivotal and pharmacokinetic studies. Circulation 2017;136:1102–13.
Crossref| PubMed - Gray WA, Jaff MR, Parikh SA, et al. Mortality assessment of paclitaxel-coated balloons: patient-level meta-analysis of the ILLUMENATE clinical program at 3 years. Circulation 2019;140:1145–55.
Crossref| PubMed - Gray WA, Keirse K, Soga Y, et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet 2018;392:1541–51.
Crossref| PubMed - Dake MD, Ansel GM, Jaff MR, et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J Am Coll Cardiol 2013;61:2417–27.
Crossref| PubMed - Holden A, Varcoe RL, Jaff MR, et al. Paclitaxel and mortality: the dose argument is critical. J Endovasc Ther 2019;26:467–70.
Crossref| PubMed - Ducasse E, Caradu C. Rigorous focus on paclitaxel-related mortality in femoropopliteal artery disease. J Vasc Surg 2020;71:216–9.
Crossref| PubMed - FDA. FDA Executive Summary: Circulatory System Devices Panel Meeting June 19 and 20, 2019. 2019. https://www.fda.gov/media/127698/download (accessed 3 June 2020).
- Dake MD, Ansel GM, Bosiers M, et al. Paclitaxel-coated Zilver PTX drug-eluting stent treatment does not result in increased long-term all-cause mortality compared to uncoated devices. Cardiovasc Intervent Radiol 2020;43:8–19.
Crossref| PubMed - Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation 2015;131:495–502.
Crossref| PubMed - Schneider PA, Varcoe RL, Secemsky E, et al. Update on paclitaxel for femoral-popliteal occlusive disease in the 15 months following a summary level meta-analysis demonstrated increased risk of late mortality and dose response to paclitaxel. J Vasc Surg 2021;73:311–22.
Crossref| PubMed