Robert S Zilinyi, Marissa Alsaloum, Aishwarya Raja, Matthew Finn, Sanjum S Sethi, Sahil Parikh
|
Abstract Below-the-knee (BTK) peripheral arterial disease (PAD) is a significant source of cardiovascular morbidity and mortality. Patients with chronic limbthreatening ischaemia in BTK PAD represent one of the highest-risk cohorts of PAD patients, with little progress having been made regarding long-term patency and mortality over the past four decades. Although conventional balloon angioplasty has long been the standard of care, several novel scaffolds have been introduced over the past decade with promising 1-year patency rates and freedom from clinically driven target limb revascularisation, which may herald a new age of endovascular intervention for BTK PAD. In this review, we present a critical appraisal of the literature surrounding novel scaffolds and drug-eluting technology in the treatment of BTK PAD. Keywords: Peripheral arterial disease, below the knee, endovascular scaffold, drug eluting technology, endovascular intervention
Disclosure: MF has received speaker honoraria from Janssen Pharmaceuticals. SSS has received honoraria from Janssen Pharmaceuticals and Chiesi, and consults for Boston Scientific, Inar and Terumo. SAP serves on advisory boards for Abbott, Boston Scientific, Cordis, Janssen, Medtronic and Philips; has received research support from Abbott, Boston Scientific, Surmodics, Shockwave Medical, TriReme, AcoTec and Veryan Medical; and reports consulting relationships with Canon, Inari, Abiomed, Penumbra and Terumo. All other authors have no conflicts of interest to declare.
Received: Accepted: Published online:
Correspondence Details: Sahil A Parikh, Columbia University Irving Medical Center and Columbia University Vagelos College of Physicians and Surgeons, 161 Fort Washington Avenue, 6th Floor, New York, NY 10032, US. E: sap2196@cumc.columbia.edu
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
|
Peripheral arterial disease (PAD) is defined by atherosclerotic narrowing of the peripheral arteries, leading to progressive ischaemia, intermittent claudication and, in some patients, chronic limb-threatening ischaemia (CLTI), frequently resulting in major amputation.1 Patients with CLTI suffer an inordinate burden of cardiovascular morbidity and mortality, with major amputation rates of up to 30% at 1 year and mortality rates of 25% and 50% at 1 and 5 years, respectively, which have not changed significantly in 40 years.2 Below the knee (BTK) PAD represents a large portion of the CLTI population given the predilection of frequently comorbid conditions, such as diabetes and chronic kidney disease, for the small artery vascular territories.3 The small vessel size and frequently long lesions of BTK PAD have made the optimal treatment of this patient population elusive from both a surgical and an increasingly endovascular perspective.4 In patients with intermittent claudication, the mainstay of therapy has been and continues to be, monitored exercise programs along with optimal medical therapy.5 However, in patients with CLTI secondary to BTK PAD, both surgical and endovascular options exist for revascularisation with the goal of improving long-term patency, limb salvage and, possibly, ultimately mortality. The best treatment strategy for any individual patient is frequently dictated by anatomy and the comorbid risk of treatment via either surgery or endovascular techniques. Frequently, endovascular techniques are chosen and, in all cases, straight in-line flow to the target angiosome is required. As such, in the absence of distal bypass targets, as is often the case, endovascular therapy of the tibial vessels may become the preferred strategy if primary patency can be preserved. The BASIL trial, performed to compare percutaneous transluminal angioplasty (PTA) with surgical revascularisation, demonstrated no significant difference in overall and amputation-free survival between angioplasty-first and surgery-first strategies in patients with CLTI.6 Further studies, including BASIL-2 (ISRCTN27728689) and BEST-CLI (NCT02060630), aim to fully delineate the optimal strategy for the treatment of patients with CLTI. The 2019 global vascular guidelines on the management of CLTI recommend an endovascular-first approach in most patients with aortoiliac and infra-inguinal CLTI, recognising that surgical revascularisation may be more appropriate for certain high-risk populations or those with severe common femoral disease.7
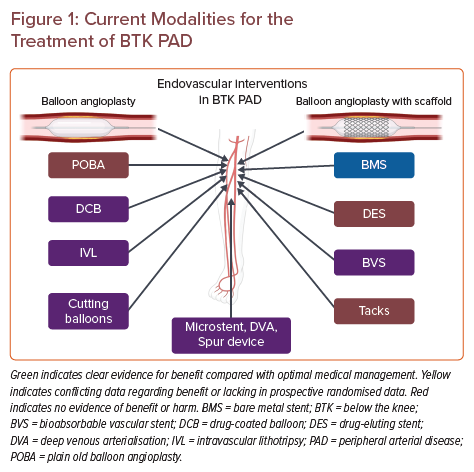
PTA has been the standard of care for the endovascular treatment of BTK PAD over the past 30 years; however, major limitations to the overall success of PTA in BTK disease include flow-limiting dissection caused by balloon dilatation and heavily calcified lesions. Vascular scaffolds, including bare metal stents (BMS), drug-eluting stents (DES), bioresorbable vascular scaffolds (BVS), and other more novel strategies have been used to treat flow-limiting dissections resulting from PTA in BTK lesions with varying levels of success. Due to the small-vessel, long-segment and multivessel nature of BTK PAD, stenting with either metallic or bioresorbable scaffolds has not been nearly as successful as it has been in the coronary, aortoiliac and femoropopliteal vasculature. However, clinical trials appraising the efficacy of scaffolds in BTK PAD are allowing for continuous iterations and improvements in patient care. In this review, we focus on the more novel scaffolds, including DES, BVS and tacks, and several newer technologies being used and studied in the BTK PAD population.
Drug-eluting Stents
The use of BMS for revascularisation in BTK PAD has been associated with poor mid-term outcomes due to in-stent restenosis, likely secondary to stent arterial wall inflammation, neointimal hyperplasia and smooth muscle cell proliferation.8 Consequently, DES technology has emerged in an attempt to prevent stent restenosis. Most DES comprise a metal stent platform covered in a polymer matrix that is saturated with an antiproliferative drug, such as sirolimus (or its analogue) or paclitaxel. In small series, the use of DES has been associated with significant improvements in the prevention of lesion restenosis and limb amputation in patients with BTK PAD.9,10
Many single- and multi-centre clinical trials have been conducted to evaluate the utility of DES in patients with CLTI secondary to BTK PAD. In one of the first such single-centre trials, 60 patients with symptomatic PAD (Rutherford Class [RC] 3–6) and angiographically proven BTK stenosis or occlusion were treated with either a BMS or sirolimus-eluting stent (SES).11 Despite the small sample size of that study, significantly favourable outcomes were observed in patients treated with the SES compared with those treated with a BMS. At a mean follow-up of approximately 10 months for both groups of patients, 7 (23.3%) patients treated with a BMS required clinically driven total lesion revascularisation (CD-TLR), compared with no patients in the SES group (p=0.0049).11 Similarly, the total number of major adverse events was lower in the SES than BMS group (3 [10%] versus 14 [46.6%], respectively; p=0.0016). At a mean angiographic follow-up time of approximately 6 months for both groups, no patients in the SES group had stent occlusion or restenosis of the treated vessel >50%, compared with 4 (17.4%; p=0.032) and 9 (39.1%; p=0.0007) patients, respectively, in the BMS group.11 In addition, the mean degree of in-stent restenosis in patients in the SES group was only 1.8%, compared with 53.0% in the BMS group (p<0.0001).11 However, due to the limited sample size and follow-up time, there was no statistically significant difference in the rate of limb amputation between the two groups, although a numerically greater number of patients in the BMS than SES group experienced a major limb amputation (3 [10%] versus 0 patients, respectively).11
Another two small single-centre randomised clinical trials were early to evaluate the efficacy of DES in BTK PAD. Falkowski et al. randomised 50 RC 3–5 patients with stenosis of one of the infrapopliteal arteries into treatment with an SES (n=25) or BMS (n=25).12 Of note, only 32% of patients included in that study had CLTI. The primary endpoint of the study was the angiographically determined restenosis rate. At the 6-month follow-up, patients in the SES group performed significantly better than the patients in the BMS group; the restenosis rate for patients in the SES group was 16% (4/25 patients), compared with 76% (19/25 patients; p<0.001) in the BMS group.12 In the BELOW study, 60 patients with CLTI were randomised to receive treatment with either PTA, abciximab plus PTA, abciximab plus a BMS, or abciximab plus an SES; after 6 months, the restenosis rates in these groups were 58%, 75%, 67% and 9%, respectively, with the SES group having the lowest restenosis rate.13
Several large multicentre trials have provided further evidence for the utility of multiple different DES in the prevention of stent restenosis after placement. One of the first of these larger multicentre trials was the Yukon-BTK trial.14,15 In that trial, 161 patients were randomised to either a polymer-free SES (n=82) or BMS (n=79). Of these patients, 75 were classified as RC 4–5. At the 1-year follow-up, there was a statistically significant difference in primary patency rate, the primary endpoint, between the two groups, favouring treatment with an SES; of the 125 patients who reached the 1-year follow-up, the primary patency rate was 80.6% (n=50) in the SES group, compared with 55.6% (n=35; p=0.004) in the BMS group.14 At a mean follow-up time of approximately 1,000 days, the event-free survival rate was 65.8% in the SES group, compared with 44.6% (p=0.02) in the BMS group.15 In addition, the rate of limb amputation was significantly lower in the SES than BMS group; 2 (5.3%) patients with CLTI who underwent SES placement experienced any type of limb amputation, compared with 7 (22.6%; p=0.04) patients who underwent BMS placement.
Alternatively, the DESTINY trial evaluated the benefit of everolimus-eluting stent (EES) placement versus BMS in the setting of CLTI.16 In that trial, 140 patients with CLTI (63 patients with RC 4 and 77 patients with RC 5) were randomised for treatment with either the Multi-Link Vision BMS (Abbott Vascular, Chicago, IL, US; n=66) or the XIENCE V DES (Abbott; n=74). The primary endpoint was primary patency at 1 year, defined as the absence of angiographically imaged in-stent restenosis ≥50%. At the 1-year follow-up, the primary patency in the EES group was 85.4% (n=50), compared with only 54.4% (n=32; p=0.0001) in the BMS group.16 Patency was superior in the EES group for patients who had both proximal and distal lesions. At the 1-year follow-up, 91.3% of patients in the EES group were free from target lesion revascularisation, compared with 66.4% in the BMS group (p=0.001). There was no difference in survival between the two groups at 1 year.16
The ACHILLES trial was another multicentre randomised clinical trial comparing SES with PTA in 200 RC 3–5 patients.17 In that study, 99 patients were randomised to the SES group and 101 were randomised to the PTA group. The primary endpoint was 1-year in-segment binary restenosis, as determined by quantitative angiography. In the intention-to-treat analysis, 41.9% of patients in the PTA group (n=31) had in-segment restenosis, compared with 22.4% (n=15) in the SES group (p=0.019).17 Statistically significant reductions in restenosis rate were also apparent in the ‘as treated’ population (p=0.004) and in the subset of patients with diabetes (p<0.001).17 Other angiographic endpoints, including percentage diameter stenosis (p=0.001) and minimal lumen diameter (p=0.044), favoured intervention with the SES.17 The clinical endpoint of vessel patency, defined as the absence of haemodynamically relevant restenosis and/or CD-TLR, also favoured treatment with the SES (n=54/72; 75.0%) over PTA (n=44/77; 57.1%; p=0.025).17 Freedom from a composite endpoint of death, target lesion revascularisation, bypass, amputation and RC ≥4 also favoured the SES over PTA group at 1 year (log-rank p=0.028).17
Finally, paclitaxel-eluting stents (PES) were compared to PTA with or without (±) BMS in the PADI trial.18 In that trial, 137 patients with CLTI (RC ≥4) were randomised to either the PES group (n=73) or the PTA ± BMS group (n=64). At the 6-month follow-up, a signal towards benefit for the PES treatment was seen, with significantly worse treatment failure noted in the PTA ± BMS than PES group, as graded by an ordinal score of both angiographic and clinical endpoints (modified intention-to-treat p=0.041).18 The lesion patency rate at 6 months showed a trend towards better outcomes in the PES group than in the PTA ± BMS group (48.0% versus 35.1%, respectively), but this did not reach statistical significance (p=0.096).18 Similarly, there was a trend towards a reduction in amputations for those treated with a PES by 2 years of follow-up, although, again, this did not reach statistical significance (p=0.066).18 At the 5-year follow-up, the differences in outcomes between patients treated with a PES or BMS became more pronounced; there was a statistically significant improvement in the composite endpoint of major amputation or death rate (p=0.043) and in the event rate per patient (p=0.041).19 The rate of amputations in patients in the PTA ± BMS group (34.0%) remained numerically higher than in the PES group (19.3%), although this, again, did not reach statistical significance (p=0.091).19 The survival rate at 5 years remained comparable between the PTA ± BMS and PTA groups (37.0% and 37.7%, respectively; p=0.45).19
In the setting of continued controversy20, 21 regarding mortality associated with paclitaxel-coated devices (discussed above), the investigators of the PADI study examined the long-term (10-year) mortality associated with PES in their cohort.22 In the PADI study, investigators noted poor mortality outcomes in both the PTA ± BMS and PES groups, with no statistically significant difference between the two groups at 10 years (log-rank p=0.12).22 Similarly, the authors reported no total dose-related mortality associated with paclitaxel (unadjusted HR 1.0, p=0.90).22 Despite the poor long-term survival of this very high-risk cohort of patients, regardless of treatment option for critical limb ischaemia, treatment with PES is theorised to offer additional economic benefits, at least through 5 years, due to the increased incidence- and event-free survival associated with the PES.23
The SAVAL trial is a recently completed multicentre trial studying a DES designed specifically for use in infrapopliteal critical limb ischaemia (NCT03551496). The SAVAL DES (Boston Scientific) is a paclitaxel-coated self-expanding stent measuring 3.5 mm × 80 mm, longer than coronary artery stents, specifically engineered to be durable in infrapopliteal arteries. The SAVAL stent was designed based on the Eluvia stent, a self-expanding PES designed and tested for use in femoropopliteal PAD.24 The SAVAL stent uses a similar dual-layer drug-coating design to that of the Eluvia with a modified paclitaxel dosing scheme specifically designed for the peripheral vascular bed.25 Eligibility criteria for the SAVAL trial include RC 4–5 disease with a target lesion at least 4 cm above the ankle joint, two or fewer infrapopliteal lesions, reference vessel diameter of 2.5–3.75 mm, life expectancy >1 year and no prior stent or surgery in the target vessel. Phase A randomised 201 subjects into receiving the SAVAL DES or PTA alone, in a 2:1 fashion, with the primary endpoint being primary patency at 12 months. Phase B involves non-randomly administering the SAVAL DES to 100 patients to gather further safety and efficacy data, with the primary outcome being the assessment of major adverse events at 12 months. Patients are being followed for 3 years, with periodic clinical and ultrasound follow-up. Preliminary data from the SAVAL trial was presented at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) 2022 annual meeting by Hans van Overhagen. Disappointingly, at 12 months, the patency of the SAVAL DES (68.0%) was not superior to PTA alone (76.0%, 95% CI for difference −22.9, 6.8%, p=0.8552). Similarly, non-inferiority analysis for major adverse events showed that the SAVAL DES was not noninferior to PTA alone; the MAE-free rate at 12 months was 91.6% in the DES cohort and 95.3% in the PTA cohort (non-inferiority p=0.0433). Despite these results, patient follow-up is continuing through three years in-office with vital status assessment through 5 years, as defined in the study protocol. It is not immediately clear why the SAVAL trial is not showing as efficacious a result as previous BTK paclitaxel-eluting stent trials (e.g. the PADI trial). Perhaps differences in the stent systems themselves may play a role in explaining this discordance. However, it is also worth noting that a significantly greater proportion of patients in the SAVAL DES group had moderate or severe calcification (57.0%) than in the PTA group (40.8%, p=0.0221). More severe lesions at baseline may falsely minimize the benefit of the stent in this patient cohort.
Bioresorbable Scaffolds
DES used in BTK PAD have shown promising results in terms of short-term patency rates, freedom from CD-TLR and major amputation, as described above. However, there is still concern over the long-term effects of indwelling metal scaffolds in small BTK arteries, specifically the ongoing nidus of arterial wall inflammation represented by the remaining scaffold long after the antiproliferative drug has been delivered.26, 27 Therefore, as with the coronary vasculature, bioresorbable scaffolds have been developed and studied in BTK PAD to provide a scaffold that maintains vessel patency after PTA and enables sustained antiproliferative drug delivery, but one that will ultimately be reabsorbed with time.
Bioresorbable scaffolds were first developed and used in coronary vasculature. Early data from the ABSORB II trial comparing an everolimus-eluting bioresorbable scaffold with the XIENCE DES (Abbott) in de novo coronary lesions were promising, with no significant difference in the composite endpoint of 1-year cardiac death, MI or target lesion revascularisation.28 However, the 3-year results of the ABSORB II trial showed significantly higher target lesion failure with the everolimus-eluting bioresorbable scaffold, and the scaffold was subsequently taken off the market for coronary indication.29
The first developed bioresorbable drug-eluting scaffold for BTK indications was reported in 2005 with the absorbable metal stent (AMS; Magic, Biotronik, Berlin, Germany). Bosiers et al. reported the 12-month outcomes of AMS use in BTK PAD.30 In that pilot study, 15 of 20 patients were RC 4–5. Procedural success was achieved in 100% of patients. The 1-year primary patency, limb salvage and survival rates were 73.3%, 94.7% and 85% respectively.30 This led to the subsequent AMS Insight trial, a large multicentre randomised trial comparing AMS with PTA alone in patients with BTK PAD and CLTI.31 In that trial, 117 patients with RC 4–5 symptoms were enrolled from across four countries in Europe and randomised (57 to PTA, 60 to AMS). Procedural success was achieved in 100% of AMS patients and 96.4% of PTA patients.31 There was no significant difference in the primary safety endpoint of 30-day freedom from major amputation or death between the two groups. Unfortunately, likely due, in part, to high rates of procedural cross-over, loss to follow-up and incomplete 6-month data, in the intention-to-treat analysis of that study, AMS failed to outperform and, in fact, was inferior to PTA alone, with 6-month primary patency rates of 31.8% in the AMS arm and 58% in the PTA-alone arm (p=0.013).31
The largest body of literature to date exists for the Absorb BVS (Abbott Vascular, Santa Clara, CA, US).32, 33 In 2016, Varcoe et al. presented the 12-month results of their prospective, single-centre experience using the Absorb BVS in BTK PAD.33 The Absorb BVS comprises a poly L-lactic acid coated with a poly(d,l-lactide) polymer that controls the release of everolimus from the BVS, which is fully resorbable. The dose of everolimus delivered by the Absorb BVS (100 µg/mm2) is equivalent to that of the XIENCE Prime DES (Abbott Vascular). The primary efficacy endpoint in the study of Varcoe et al. was the freedom from binary restenosis rate, defined by a peak systolic velocity ratio >2.0.33 Secondary endpoints included CD-TLR, amputation, bypass surgery, cardiovascular and all-cause mortality and ‘any related morbidity within 30 days of the index procedure’.33 Primary patency was defined by freedom from CD-TLR and binary restenosis. In all, 33 patients with severe claudication to CLTI (RC 3–5) were enrolled (68.4% CLTI, 31.6% severe claudication); 50 scaffolds were used to treat 43 distinct lesions, with 34% of patients receiving treatment of inflow lesions either preceding their BVS procedure or concomitantly. Procedural success, defined by the successful deployment of the Absorb BVS with <30% residual stenosis and no evidence of acute thrombosis, was achieved in 100% of patients.33 One patient underwent CD-TLR on day 2 after the procedure and was found to have occlusions in two of the three BVSs placed. Three patients developed femoral pseudoaneurysms during follow-up; two of these patients required covered stent placement and one required open surgical repair. Twelve-month survival was 84.8%. Freedom from CD-TLR was 96% at 6, 12 and 24 months. Primary patency was 96%, 96% and 84.6% at 6, 12 and 24 months respectively. Seventy-nine per cent of patients showed clinical improvement, defined as either wound healing or improvement in RC. Sixty-four per cent of patients with RC 5–6 had complete healing of their wounds during the follow-up period.33 Varcoe et al. have recently reported the 5-year outcomes of these data with an additional 15 patients added to their series for a total of 48 patients with 71 scaffolds in 61 lesions.34 Impressively, the authors report binary restenosis in only 15.5% of BVS at 5 years, primary patency in 72.3% at 5 years and freedom from CD-TLR of 90.7% at 5 years.34 After a mean follow-up period of 35.2±20.4 months, 54.2% of patients were alive.34
In a 2019 retrospective case series, Dia et al. reported the results of their institutional experience with the Absorb BVS in BTK PAD, demonstrating 100% procedural success with 1-year freedom from clinically driven target vessel failure of 95.1%, primary patency of 96.7%, improvement in RC in 96.8% of patients and no deaths at 1 year.32 In 2020, Kum et al. subsequently reported the results of the DISAPEAR registry, a retrospective registry of Asian patients treated with Absorb BVS in BTK PAD.35 In 41 patients with CLTI due to BTK PAD, the authors reported 100% technical success, 6- and 12-month primary patency of 95% and 86%, respectively, 1-year freedom from CD-TLR and major amputation of 93% and 98%, respectively, and amputation-free survival of 85% at 1 year.35 Seventy-nine per cent of patients with RC 5–6 symptoms had wound healing by 12 months.35
In 2021, Huizing et al. published a pooled analysis of three separate real-world cohorts using the ABSORB BVS in BTK PAD.36 The primary endpoint in this analysis was freedom from restenosis, with secondary endpoints of freedom from CD-TLR, major amputation and survival. In all, 121 patients received 189 ABSORB BVS, with 75% of patients in RC 5–6. The primary endpoint of freedom from restenosis was achieved in 91.7% and 86.6% of patients at 12 and 24 months, respectively.36 Freedom from CD-TLR was 97.2% and 96.6% at 12 and 24 months, respectively, with major amputation in only 1.6% of limbs at 24 months. Overall survival was 85% at 24 months.36
Ipema et al. recently published a systemic review and meta-analysis of the existing literature surrounding bioresorbable scaffolds in BTK PAD with 12-month data available. That analysis indicated a pooled 1-year primary patency rate of 90%, freedom from CD-TLR of 96%, limb salvage rate of 97% and survival of 90%.37
These data are compelling and offer the first potentially tenable solution to the question of how to deliver antiproliferative drugs to target vessels in BTK PAD, maintaining left main patency with a vascular scaffold while avoiding the negative long-term effects of metallic scaffolds. Although compelling, the data apart from Insight AMS all come from non-randomised prospective and retrospective case series. The ongoing LIFE-BTK trial (NCT04227899) is a prospective randomised multicentre global trial assessing the safety and efficacy of the Esprit bioresorbable scaffold in BTK PAD compared with PTA. We eagerly await the results of this trial to better define the role of bioresorbable scaffolds in the management of BTK PAD.
Tacks
In this section, we discuss the most recent addition to the armamentarium of tools for the treatment of BTK PAD, the Tack Endovascular System. Flow-limiting dissections remain one of the primary limitations to successful PTA and often necessitate the placement of an endovascular scaffold to maintain vessel patency. As discussed in the preceding sections, deploying vascular scaffolds in BTK arteries has been fraught with low long-term patency rates with BMS, concerns regarding long-term failure secondary to external crush forces on repurposed coronary DES and a lack of prospective randomised data in the area of bioresorbable scaffolds. The Tack endovascular system uses short (<6–10 mm)-segment, open-cell metallic stents to tack up dissection flaps to maintain vessel patency after PTA. The Tack Endovascular System for BTK lesions uses a 4 Fr system with four independent Tack implants (Figure 1) for vessels ranging in diameter from 1.5 to 4.5 mm.
The 6-month data of the TOBA II BTK study, in which the Tack Endovascular System was used for the treatment of angiographically confirmed dissections after PTA in BTK arteries, were published in 2020.38 In that study, 233 patients with RC 3–5 symptoms were enrolled. The primary safety endpoint was a composite of 30-day major adverse limb events (MALE) and all-cause postoperative death (POD). The primary efficacy endpoint was a composite 6-month of MALE and 30-day POD. These same endpoints were evaluated at 12 months, along with amputation-free survival (AFS), freedom from CD-TLR, vessel patency, and changes in clinical and quality-of-life-measures. The authors reported impressive results, with 93.4% of patients free of the composite endpoint of MALE and POD, a tacked segment patency of 81.3%, a limb salvage rate of 96.8%, freedom from CD-TLR of 83.1% and AFS of 89.3%.38 In addition, 82.4% and 72.5% of patients had sustained improvements in RC and in wound healing, respectively. This trial’s 6-month data led to the US Food and Drug Administration’s (FDA) approval of this device in BTK PAD, the first vascular scaffold to receive an indication in BTK PAD.
Devices in Development
The landscape of endovascular interventions for BTK PAD continues to change rapidly. In this section, we review the current therapies in various stages of development and how they may fit into the treatment paradigm for BTK PAD. Ongoing trials with a novel scaffold for BTK PAD are described in Supplementary Material Table 1.
Micro Medical Solutions has developed an integrated platform of microstent, microcatheter and microballoon specifically designed for use in the BTK PAD population. The microstent, a self-expanding woven nitinol stent, is designed to conform to complex tibial lesions and exert lower radial force than balloon-expandable stents while maintaining luminal gain and vessel patency after PTA. The FDA granted investigational device exemption status to the MICROSTENT platform in 2019 following the report of its safety and feasibility study in 15 patients across three centres with RC 4–5 symptoms.39 One hundred per cent of patients were free from the primary safety endpoint of MALE or POD at 30 days, and 91.7% of patients maintained primary patency at 30 days, with 100% of patients free from CD-TLR (unpublished data; presented by Robert Beasley at the Leipzig Interventional Course in 2019). These results were carried out to 6 months, with 90.9% of patients achieving primary patency, ultimately paving the way for the ongoing STAND randomised trial comparing the microstent with standard PTA in BTK PAD (NCT03477604). This trial aims to enrol 177 participants with RC 4–5 BTK PAD across multiple centres in the US and to examine primary patency, defined as freedom from target vessel occlusion, CD-TLR or major amputation in the target limb, at 6 months. Primary safety endpoints are freedom from MALE at 6 months and POD at 30 days. Stenting has largely been reserved as a bailout strategy to treat recoil and flow-limiting dissections in BTK PAD. This is notably one of the first contemporary randomised trials in this population comparing primary stenting with standard of care PTA. If the primary patency rates seen in the feasibility study of the microstent carry over to the STAND trial, this may prove to be a paradigm shift for the field of BTK PAD, particularly if these patency rates are maintained in the long term.
Reflow Medical has developed the Spur device for use in the BTK distribution, specifically in combination with antiproliferative drug therapy. One of the principal issues with drug-eluting technology in BTK PAD is achieving adequate vessel preparation to facilitate maximum vessel surface contact with the drug-eluting device and adequate penetration of the antiproliferative drug into the vessel wall. The Spur device uses a temporary stent with radial spikes designed to penetrate atherosclerotic lesions to reach deeper layers of the arterial wall to facilitate acute luminal gain and delivery of the antiproliferative drug. The DEEPER LIMUS trial is an ongoing pilot study conducted on 30 patients in a single centre in Germany assessing the safety of this device in people with BTK PAD (NCT04162418). The primary endpoint in that study is a 6-month composite endpoint of all-cause mortality, freedom from CD-TLR and major amputation. Secondary efficacy endpoints include 6-month late lumen loss, primary patency, change in RC and wound healing. The FDA has given the Spur device Breakthrough Device designation. This designation facilitates the speedy review of devices with the potential to offer more effective treatment of life-threatening or permanently debilitating diseases.
The final device we discuss is the LimFlow stent graft system (LimFlow), designed to achieve percutaneous deep vein arterialisation (DVA) in no-option BTK PAD patients, which is defined as RC 5–6 deemed by a team of vascular surgeons and interventionalists to have no percutaneous or surgical bypass options for their level of disease. The procedure uses simultaneous retrograde pedal access to the desired deep vein and antegrade arterial access to the diseased tibial vessel. A valvulotome is used to render the vein distal to the diseased artery incompetent, after which an iatrogenic atrioventricular (AV) fistula is created proximal to the level of disease in the target artery. This AV fistula is then lined with polytetrafluoroethylene-covered stents, creating an in-line flow to the pedal vessels with the goal of improving wound healing, limb salvage and symptom improvement. In 2019, Mustapha et al. reported the interim results of the PROMISE I trial, a single-arm multicentre pilot study examining the safety and efficacy of the LimFlow stent graft system in 10 patients with no-option critical limb ischaemia.40 The primary and secondary safety endpoints were AFS at 30 days and 6 months, respectively. Secondary efficacy endpoints included primary patency, wound healing and technical success. The authors reported 100% AFS at both 30 days and 6 months, with a 100% technical success rate and no reported procedural complications.40 The 1- and 6-month primary patency rates were 90% and 40%, respectively with 30% of patients requiring reintervention. At 6 months, 80% of patients had >60% wound healing.40 In early 2021, Clair et al. reported 12-month data from the full PROMISE I cohort, including 32 patients across seven sites in the US treated with the LimFlow stent graft system.41 The authors reported a 97% technical success rate, and 30-day, 6-month and 12-month AFS rates of 91%, 74% and 70%, respectively. Seventy-five per cent of wounds were healed or healing at 12 months. Fifty-two per cent of patients required reintervention, predominantly driven by inflow disease proximal to the DVA circuit.41 At 24 months, the AFS rate was 59%, driven by an overall increase in all-cause mortality with a stable rate of freedom from major amputation.41 Eighty-five per cent of patients had fully healed wounds.41 The encouraging results of the PROMISE I trial have led to the initiation of a larger PROMISE II trial, which is being conducted at 22 sites across the US and Japan, enrolling 120 patients with no-option BTK PAD.
References
- Jones DW, Farber A. Review of the global vascular guidelines on the management of chronic limb-threatening ischemia. JAMA Surg 2020;155:161–2. Crossref | PubMed
- Mustapha JA, Katzen BT, Neville RF, et al. Disease burden and clinical outcomes following initial diagnosis of critical limb ischemia in the medicare population. JACC Cardiovasc Interv 2018;11:1011–2. Crossref | PubMed
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45(Suppl S):S5–67. Crossref | PubMed
- Shishehbor MH. Endovascular treatment of femoropopliteal lesions: so many options, little consensus. J Am Coll Cardiol 2015;66:2339–42. doi:doi. Crossref | PubMed
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation 2017;135:e686–725. doi:doi. Crossref | PubMed
- Bradbury AW, Adam DJ, Bell J, et al. Bypass versus angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a survival prediction model to facilitate clinical decision making. J Vasc Surg 2010;51(5 Suppl):52S–68. Crossref | PubMed
- Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019;69(6 Suppl):3S–125S.e40. Crossref | PubMed
- Epstein SE, Speir E, Unger EF, et al. The basis of molecular strategies for treating coronary restenosis after angioplasty. J Am Coll Cardiol 1994;23:1278–88. Crossref | PubMed
- Zhang J, Xu X, Kong J, et al. Systematic review and meta-analysis of drug-eluting balloon and stent for infrapopliteal artery revascularization. Vasc Endovasc Surg 2017;51:72–83. Crossref | PubMed
- Almasri J, Adusumalli J, Asi N, et al. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;58:S110–9. Crossref | PubMed
- Scheinert D, Ulrich M, Scheinert S, et al. Comparison of sirolimus-eluting vs. bare-metal stents for the treatment of infrapopliteal obstructions. EuroIntervention 2006;2:169–74.
- Falkowski A, Poncyljusz W, Wilk G, Szczerbo-Trojanowska M. The evaluation of primary stenting of sirolimus-eluting versus bare-metal stents in the treatment of atherosclerotic lesions of crural arteries. Eur Radiol 2009;19:966–74. Crossref | PubMed
- Tepe G, Schmehl J, Heller S, et al. Drug eluting stents versus PTA with GP IIb/IIIa blockade below the knee in patients with current ulcers–the below Study. J Cardiovasc Surg (Torino) 2010;51:203–12.
- Rastan A, Tepe G, Krankenberg H, et al. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J 2011;32:2274–81. Crossref | PubMed
- Rastan A, Brechtel K, Krankenberg H, et al. Sirolimus-eluting stents for treatment of infrapopliteal arteries reduce clinical event rate compared to bare-metal stents: long-term results from a randomized trial. J Am Coll Cardiol 2012;60:587–91. Crossref | PubMed
- Bosiers M, Scheinert D, Peeters P, et al. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg 2012;55:390–8. Crossref | PubMed
- Scheinert D, Katsanos K, Zeller T, et al. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol 2012;60:2290–5. Crossref | PubMed
- Spreen MI, Martens JM, Hansen BE, et al. Percutaneous transluminal angioplasty and drug-eluting stents for infrapopliteal lesions in critical limb ischemia (PADI) trial. Circ Cardiovasc Interv 2016;9:e002376. Crossref | PubMed
- Spreen MI, Martens JM, Knippenberg B, et al. Long-term follow-up of the PADI trial: percutaneous transluminal angioplasty versus drug-eluting stents for infrapopliteal lesions in critical limb ischemia. J Am Heart Assoc 2017;6:e004877. Crossref | PubMed
- Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death and amputation with use of paclitaxel-coated balloons in the infrapopliteal arteries for treatment of critical limb ischemia: a systematic review and meta-analysis of randomized controlled trials. J Vasc Interv Radiol 2020;31:202–12. Crossref | PubMed
- Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7:e011245. Crossref | PubMed
- Konijn LCD, Wakkie T, Spreen MI, et al. 10-year paclitaxel dose-related outcomes of drug-eluting stents treated below the knee in patients with chronic limb-threatening ischemia (the PADI trial). Cardiovasc Intervent Radiol 2020;43:1881–8. Crossref | PubMed
- Wakkie T, Konijn LCD, van Herpen NPC, et al. Cost-effectiveness of drug-eluting stents for infrapopliteal lesions in patients with critical limb ischemia: the PADI trial. Cardiovasc Intervent Radiol 2020;43:376–81. Crossref | PubMed
- Müller-Hülsbeck S, Keirse K, Zeller T, et al. Twelve-month results from the MAJESTIC trial of the eluvia paclitaxel-eluting stent for treatment of obstructive femoropopliteal disease. J Endovasc Ther 2016;23:701–7. Crossref | PubMed
- Kokkinidis DG, Armstrong EJ. Current developments in endovascular therapy of peripheral vascular disease. J Thorac Dis 2020;12:1681–94. Crossref | PubMed
- Grewe PH, Deneke T, Machraoui A, et al. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol 2000;35:157–63. Crossref | PubMed
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193–202. Crossref | PubMed
- Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2015;385:43–54. Crossref | PubMed
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388:2479–91. Crossref | PubMed
- Bosiers M, Deloose K, Verbist J, Peeters P. First clinical application of absorbable metal stents in the treatment of critical limb ischemia: 12-month results. Vasc Dis Manag 2005;2:86–91.
- Bosiers M, Peeters P, D’Archambeau O, et al. AMS Insight –absorbable metal stent implantation for treatment of below-the-knee critical limb ischemia: 6-month analysis. Cardiovasc Intervent Radiol 2009;32:424–35. Crossref | PubMed
- Dia A, Venturini JM, Kalathiya R, et al. Single arm retrospective study of bioresorbable vascular scaffolds to treat patients with severe infrapopliteal arterial disease. Catheter Cardiovasc Interv 2019;94:1028–33. Crossref | PubMed
- Varcoe RL, Schouten O, Thomas SD, Lennox AF. Experience with the absorb everolimus-eluting bioresorbable vascular scaffold in arteries below the knee: 12-month clinical and imaging outcomes. JACC Cardiovasc Interv 2016;9:1721–8. Crossref | PubMed
- Varcoe RL, Menting TP, Thomas SD, Lennox AF. Long-term results of a prospective, single-arm evaluation of everolimus-eluting bioresorbable vascular scaffolds in infrapopliteal arteries. Catheter Cardiovasc Interv 2021;97:142–9. Crossref | PubMed
- Kum S, Ipema J, Chun-Yin DH, et al. Early and midterm experience with the absorb everolimus-eluting bioresorbable vascular scaffold in Asian patients with chronic limb-threatening ischemia: one-year clinical and imaging outcomes from the DISAPEAR registry. J Endovasc Ther 2020;27:616–22. Crossref | PubMed
- Huizing E, Kum S, Ipema J, et al. Mid-term outcomes of an everolimus-eluting bioresorbable vascular scaffold in patients with below-the-knee arterial disease: a pooled analysis of individual patient data. Vasc Med 2021;26:195–9. Crossref | PubMed
- Ipema J, Kum S, Huizing E, et al. A systematic review and meta-analysis of bioresorbable vascular scaffolds for below-the-knee arterial disease. Int Angiol 2021;40:42–51. Crossref | PubMed
- Geraghty PJ, Adams G, Schmidt A. Six-month pivotal results of tack optimized balloon angioplasty using the Tack Endovascular System in below-the-knee arteries. J Vasc Surg 2021;73:918-929.e5. Crossref | PubMed
- Micro Medical solutions receives FDA IDE approval for pivotal clinical trial of MicroStent. Cath Lab Digest. 24 June 2019. (accessed 18 July 2022).
- Mustapha JA, Saab FA, Clair D, Schneider P. Interim results of the PROMISE I Trial to investigate the LimFlow system of percutaneous deep vein arterialization for the treatment of critical limb ischemia. J Invasive Cardiol 2019;31:57–63. PubMed
- Clair DG, Mustapha JA, Shishehbor MH, et al. Promise I: Early feasibility study of the LimFlow System for percutaneous deep vein arterialization in no-option chronic limb-threatening ischemia: 12-month results. J Vasc Surg 2021;74:1626–35. Crossref | PubMed
