Luca Scott, Jack Cullen
|
Abstract Pelvic vein embolisation (PVE) with metallic coils is an effective treatment for pelvic venous congestion. The migration of coils following the procedure has been well-reported; however, the most effective approach to management is still unclear. In the present case, the authors describe the delayed identification of a migrated coil to the right ventricle following an ovarian vein embolisation. The patient presented to the emergency department with chest pain and subsequent radiology identified a coil in the right ventricle. This was found to be present on previous radiology, but had not been reported on. The position of the coil had remained stable and therefore was deemed an unlikely cause for the chest pain. The coil was managed conservatively. This demonstrates how asymptomatic coil migration may go undetected and thus the migration rates in the literature may be underreported. Post-PVE screening to assess for migration could improve the accuracy of complication rates and prevent delayed complications associated with migrated coils. Keywords: Pelvic vein embolisation, coil migration, venous congestion Disclosure: The authors have no conflicts of interest to declare. Received: Accepted:Published online: Support: Informed consent: The patient provided informed consent on 25 August 2020 to write this case report. Acknowledgements: The authors thank Mr Stephen Black for his expertise and guidance on the scope of the article. Correspondence Details: Luca Scott, Guy’s and St Thomas’ Hospital, Westminster Bridge Road, London SE1 7EH, UK. E: lucascott@doctors.org.uk Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
|
Case Report
A 54-year-old woman presented to the emergency department with sudden-onset right-sided pleuritic chest pain with associated shortness of breath. Her past medical history consisted of autoimmune thyroiditis, previous pancreatitis and a complex vascular history requiring several interventions. The patient’s chronic lower limb varicosities were treated with a long saphenous vein laser ablation and foam sclerotherapy. Her chronic pelvic pain associated with pelvic congestion syndrome had been managed with an ovarian iliac vein embolisation in 2014, which was repeated in 2019 due to persistent reflux through the original coils.
On presentation the patient was tachycardic with a heart rate of 100 BPM, otherwise her observations were unremarkable. A chest X-ray and CT pulmonary angiogram (CTPA) were ordered to investigate primarily for a pulmonary embolism as well as for other differentials of her presentation, such as pneumothoraces and pneumonias.
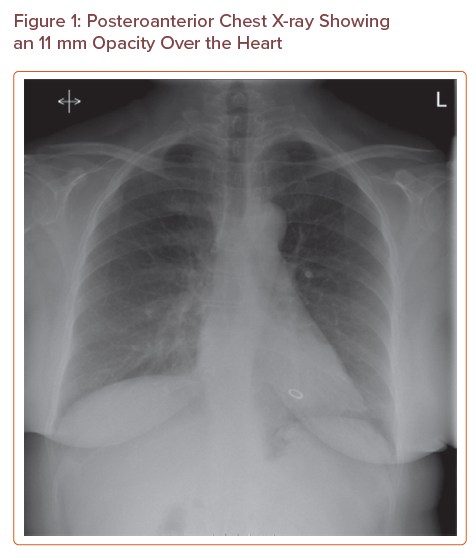
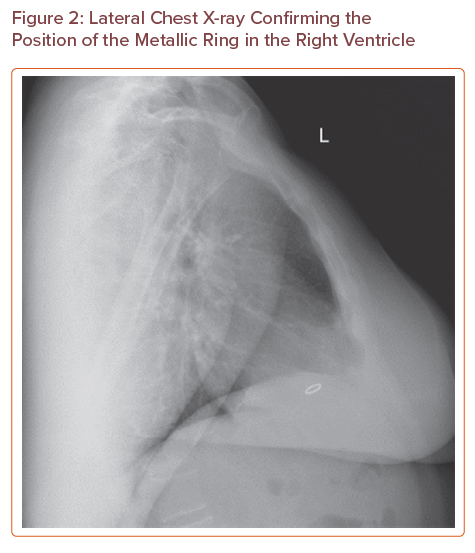
The chest X-ray (Figure 1) showed an 11 mm metallic ring opacity overlying the ventricles; this was at first queried as an artefact. Further examination of the patient revealed no overlying jewellery or clothing that could explain the radiological appearance. A lateral X-ray (Figure 2) confirmed the presence of the metallic ring in the right ventricle.
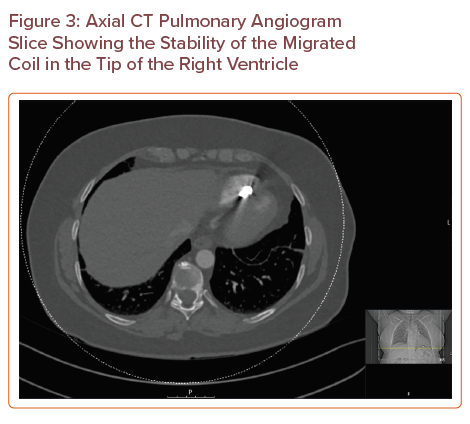
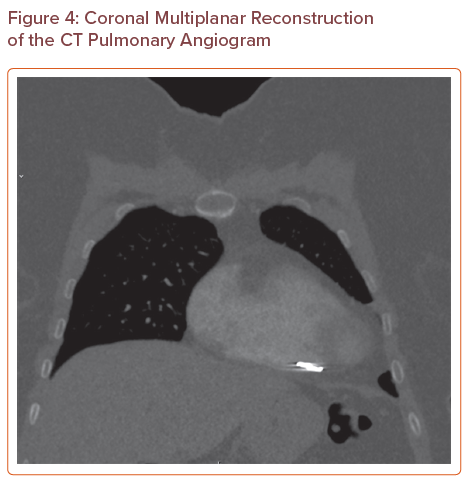
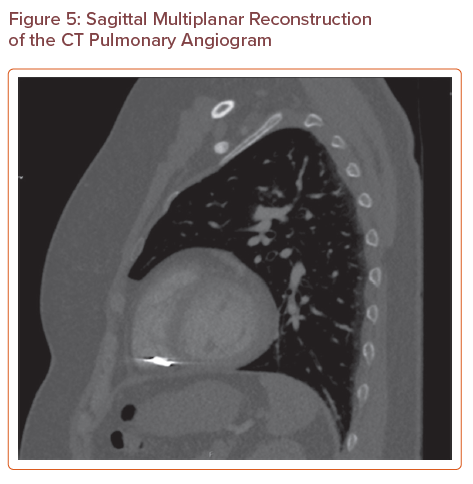
A CTPA (Figures 3, 4 and 5) showed that there was no pulmonary embolus or right heart strain, and confirmed the presence of an 11 mm metallic foreign body at the tip of the right ventricle, which was suspected to be a migrated pelvic vein embolisation (PVE) coil. On further investigation, the coil had been present on a previous CT of the abdomen and pelvis that had been requested for severe abdominal pain in 2016, but its presence had not been discussed in the radiologist’s report. The position of the coil had remained stable.
The patient was referred to the cardiac surgeons, who performed a bedside echocardiogram and found that the coil had endothelialised into the cardiac septum. The foreign body was considered to be a low risk for thrombus, and therefore no intervention or anticoagulation was required. ECG showed a normal sinus rhythm, and blood tests including troponin were unremarkable. The chest pain was deemed to be musculoskeletal in nature given that no acute cause was identified.
The patient was discharged and her case discussed in the vascular multidisciplinary meeting, which concluded that there was no interval change in appearance of the migrated coil, and therefore it was unlikely to be the cause of the patient’s symptoms. Similarly, an outpatient cardiology review concluded that the coil was both unlikely to be the cause of the patient’s symptoms or to result in future complications.
Discussion
Chronic pelvic pain is a complex condition that can be the result of gynaecological diseases, such as pelvic inflammatory disease, endometriosis and fibroids. Alternatively, referred pain, psychological factors and venous incompetence are known to also contribute. In up to 60% of patients no definitive aetiology for chronic pelvic pain is identified.1,2 Pelvic venous congestion (PVC) is a cause of persistent non-cyclic chronic pelvic pain, typically as the result of ovarian vein incompetence resulting in pelvic varicosities. The pain is often exacerbated by standing and intercourse. Multiparous and premenopausal women are at greater risk of developing PVC and its prevalence is in the range of 15–30%.1–3 PVC can be managed conservatively with analgesia, through surgical ligation or alternatively with interventional PVE.
Posteroanterior Chest X-ray Showing an 11 mm Opacity Over the Heart
Lateral Chest X-ray Confirming the Position of the Metallic Ring in the Right Ventricle
Axial CT Pulmonary Angiogram Slice Showing the Stability of the Migrated Coil in the Tip of the Right Ventricle
PVE has been shown to be an effective treatment in women with chronic pelvic pain resulting from pelvic venous disorders.1,3 The embolic agents used can vary between coils, glue, vascular plugs, foam and liquid sclerosants, with some clinicians advocating the combined use of coils and sclerosing agents. Current clinical evidence is insufficient to compare the outcomes between the use of coils alone and in combination.1,4 Arterial percutaneous embolisation with coils was first used effectively in 1975, and in 1993 Edwards et al. documented the first use of coil embolisation in the management of PVC.5 PVE has been found to be efficacious in the management of PVC, with improvement in symptoms seen in 70–90% of patients.3,6
The complications associated with PVE include bleeding, venous perforation and coil migration. Coil migration can be an immediate or delayed complication. Common migration sites include the pulmonary vasculature and the heart. Several studies have investigated the effectiveness of PVE in pelvic congestion syndrome and identified the risks of coil displacement. Kwon et al. demonstrated an 82% reduction in pain in 67 patients, but a 3% risk of coil embolization.7 Similarly, Ratnam et al. found a 1.4% risk of coil migration in 218 patients undergoing PVE.1,8 A systematic review of the effectiveness PVE in 1,308 patients across 22 studies reported a substantial early improvement in pain in approximately 75% of patients and a coil migration risk of <2%.9
Interestingly, in the present case, the migrated coil was an incidental finding in a symptomatic patient and may not have been discovered had the patient not presented to the emergency department with chest pain. The stable appearance and asymptomatic nature of the migrated coil in the 4 year interval between the CT of the abdomen and pelvis in 2016 and the CTPA in 2020 mean that the chest pain is unlikely to be related to the coil. This raises the possibility that asymptomatic migrated coils may go underreported following PVE. The current coil migration rates in the literature relate predominantly to symptomatic patients or to those presenting with complications in which investigations have noted a migrated coil. Thus, many patients who have had PVE may have migrated coils after the procedure, and the absence of associated symptoms mean that the migrated coils go undiscovered. Patients in this group could present at a later date with complications of coil migration, such as arrhythmias, thrombus, cardiac valve dysfunction or haemopericardium.2,3,10
To the best of our knowledge, the underreporting of coil migration rates has not previously been discussed in the literature. Post-PVE screening at 6 weeks could help to prevent future complications of undetected migrated coils and improve the accuracy of documented migration rates. If true, underreporting also highlights the preoperative issue of incorrect complication rates being discussed when obtaining patient consent. The heart and pulmonary vasculature are common sites of migration. Therefore, a plain film chest radiograph, given the associated minimal radiation exposure to the patient, may be a sufficient screening tool for this purpose.
Coil migration following PVE has been well-reported in the literature, however, the management of displaced coils often differs between cases. In a similar respect to the present case; there are reports of coil migration to the right side of the heart both with and without methods of retrieval. Kyaw et al. outlined a case of coil migration to the right atrium immediately after a PVE procedure for pelvic congestion syndrome.10 Several unsuccessful percutaneous attempts were made at coil retrieval and thus their patient had to undergo an open thoracotomy in order for the coil to be removed from the damaged tricuspid valve.10 A further paper by Rastogi et al. discussed a case of coil migration following bilateral ovarian vein embolisation to the right ventricle.2 Their patient remained haemodynamically stable and asymptomatic throughout and it was decided that no further treatment was required.2
In the present case, the patient had remained asymptomatic for several years after her PVE and the migrated coil was an incidental radiological finding. The endothelialised coil was believed to pose a low risk for thrombus and thus anticoagulation was not considered. Tonkin and Madden chose to anticoagulate in one of the three cases of coil migration they saw following PVE, advocating that anticoagulation should be considered only if there is an associated thrombus or an increased thrombotic risk due to position of the coil, for example within the valve apparatus.11
To prevent complications of PVE, the importance of characterising the vessels prior to embolisation is highlighted in the literature. The type of vessel (artery or vein), the volume of blood flow through the target vessel and the size of the coil can all affect the likelihood of migration. To optimise stability, it is recommended that the coil should be greater than the diameter of the embolised vein. Yamasaki et al. suggest a coil size 30–50% greater than the target vessel, with alternative literature advocating the use of an exact coil size 1–3 mm greater than the diameter of the vessel.8,12–14 To prevent coil migration, we are reliant on the frictional resistance of the vessel and the radial force exerted by the coil and thus it is important to note the variable calibre of target veins.8,12–14 Typically, 0.035–0.038 inch coils are used with diameters ranging between 5 and 20 mm and lengths of 7–14 cm, and the number of coils deployed varies between clinicians.1,4,7
To achieve permanent vessel occlusion the coils cause a mechanical obstruction, inducing thrombosis and eventual sclerosis of the target vessels.14 The choice of materials used to manufacture coils has progressed over time, primarily from stainless steel to platinum. Coils can be enlaced with fibres to increase their thrombogenic qualities, with materials such as PET and nylon enabling more rapid vessel occlusion and the use of fewer coils.1,15,16 Further technological advances have resulted in bioactive coils. Platinum coils can be coated with a hydrogel polymer that facilitates an increase in the coil’s thickness of up to fourfold its size when it comes into contact with blood or a liquid. Expansion of coils can result in more reliable vessel occlusion in the absence of effective coagulation.17,18 In addition to the material used to produce a coil, the configuration and transformation of a material from a primary to a tertiary structure helps to determine other qualities of the coil, such as stiffness, length and diameter.19
Coronal Multiplanar Reconstruction of the CT Pulmonary Angiogram
Sagittal Multiplanar Reconstruction of the CT Pulmonary Angiogram
Chest pain is one of the most common presenting complaints seen in the emergency department and it accounts for 25% of emergency medical admissions in England and Wales.20 The aetiology of chest pain can be characterised into cardiovascular causes (acute coronary syndromes, pulmonary emboli and aortic dissections), respiratory causes (pneumothoraces, pneumonia and pleurisy), musculoskeletal causes (costochondritis and chest wall injuries) and finally gastrointestinal causes (gastro-oesophageal reflux).20 In the present case, we suspect that the patient’s chest pain was musculoskeletal in nature. The absence of pulmonary emboli on CTPA, an ECG showing a normal sinus rhythm and unremarkable troponin and inflammatory markers helped to exclude many of the aforementioned life-threatening causes of chest pain. With regards to her chest pain specifically, the patient was discharged with safety-netting advice and simple analgesia.
Conclusion
PVE has been shown to be an effective treatment for PVC. Although rare, coil migration must be considered as a possible complication of the procedure. Several case reports including the present one have demonstrated the migration of coils into the heart and the pulmonary vasculature. The presence of symptoms as a result of the migration, the thrombotic risk posed by the coil and the potential for damage to the viscera will all influence decisions regarding the need for further management of a migrated coil. In the literature, conservative approaches, as well as interventional retrieval have both been successful in the management of migrated coils.2,3,10
The present case illustrates how a migrated coil can remain asymptomatic for several years, and suggests that there may be underreporting of migration rates in the literature and discussion of inaccurate complication rates when obtaining patient consent for PVE. Screening following PVE may be a useful tool to identify migrated coils, improve the accuracy of coil migration rates and prevent late complications associated with migrated coils.
References
- Lopez AJ. Female pelvic vein embolization: indications, techniques, and outcomes. Cardiovasc Intervent Radiol 2015;38:806–20.
Crossref| PubMed - Rastogi N, Kabutey NK, Kim D. Unintended coil migration into the right ventricle during the right ovarian vein coil embolization. Vasc Endovasc Surg 2011;45:660–4.
Crossref| PubMed - Guerrero A, Theophanous RG. A case report of a migrated pelvic coil causing pulmonary infarct in an adult female. Clin Pract Cases Emerg Med 2020;4:436–9.
Crossref| PubMed - Guirola JA, Sánchez-Ballestin M, Sierre S, et al. A randomized trial of endovascular embolization treatment in pelvic congestion syndrome: fibered platinum coils versus vascular plugs with 1-year clinical outcomes. J Vasc Interv Radiol 2018;29:45–53.
Crossref| PubMed - Edwards RD, Robertson IR, MacLean AB, Hemingway AP. Case report: pelvic pain syndrome – successful treatment of a case by ovarian vein embolization. Clin Radiol 1993;47:429–31.
Crossref| PubMed - Laborda A, Medrano J, de Blas I, et al. Endovascular treatment of pelvic congestion syndrome: visual analog scale (VAS) long-term follow-up clinical evaluation in 202 patients. Cardiovasc Intervent Radiol 2013;36:1006–14.
Crossref| PubMed - Kwon SH, Oh JH, Ko KR, et al. Transcatheter ovarian vein embolization using coils for the treatment of pelvic congestion syndrome. Cardiovasc Intervent Radiol 2007;30:655–61.
Crossref| PubMed - Ratnam LA, Marsh P, Holdstock JM, et al. Pelvic vein embolisation in the management of varicose veins. Cardiovasc Intervent Radiol 2008;31:1159–64.
Crossref| PubMed - Daniels JP, Champaneria R, Shah L, et al. Effectiveness of embolization or sclerotherapy of pelvic veins for reducing chronic pelvic pain: a systematic review. J Vasc Interv Radiol 2016;27:1478–86.
Crossref| PubMed - Kyaw H, Park W, Rodriguez C, et al. Coil embolization to the right side of the heart after elective hypogastric vein embolization requiring open-heart surgery. Cath Lab Digest 2018;26(9). https://www.cathlabdigest.com/article/Coil-Embolization-Right-Side-Heart-After-Elective-Hypogastric-Vein-Embolization-Requiring (accessed 25 May 2021).
- Tonkin J, Madden B. From ovarian coils to pulmonary emboli. Am J Respir Crit Care Med 2018:197;A3700.
- Yamasaki W, Kakizawa H, Ishikawa M, et al. Migration to the pulmonary artery of nine metallic coils placed in the internal iliac vein for treatment of giant rectal varices. Acta Radiol Short Rep 2012;1:1–4.
Crossref| PubMed - Nasser F, Cavalcante RN, Affonso BB, et al. Safety, efficacy, and prognostic factors in endovascular treatment of pelvic congestion syndrome. Int J Gynaecol Obstet 2014;125:65–8.
Crossref| PubMed - Knuttinen MG, Xie K, Jani A, et al. Pelvic venous insufficiency: imaging diagnosis, treatment approaches, and therapeutic issues. AJR Am J Roentgenol 2015;204:448–58.
Crossref| PubMed - Trerotola SO, Pressler GA, Premanandan C. Nylon fibered versus non-fibered embolization coils: comparison in a swine model. J Vasc Interv Radiol 2019;30:949–55.
Crossref| PubMed - Liebig T, Henkes H, Fischer S, et al. Fibered electrolytically detachable platinum coils used for the endovascular treatment of intracranial aneurysms. Initial experiences and mid-term results in 474 aneurysms. Interv Neuroradiol 2004;10:5–26.
Crossref| PubMed - López-Benítez R, Hallscheidt P, Kratochwil C, et al. Protective embolization of the gastroduodenal artery with a one-HydroCoil technique in radioembolization procedures. Cardiovasc Intervent Radiol 2013;36:105–10.
Crossref| PubMed - Zander T, Medina S, Montes G, et al. Endoluminal occlusion devices: technology update. Med Devices (Auckl) 2014;7:425–36.
Crossref| PubMed - White JB, Ken CG, Cloft HJ, Kallmes DF. Coils in a nutshell: a review of coil physical properties. AJNR Am J Neuroradiol. 2008;29:1242–6.
Crossref| PubMed - Kendall J, Hancock I. Chest pain syndromes. London: Royal College of Emergency Medicine, 2019. https://www.rcemlearning.co.uk/reference/chest-pain-syndromes (accessed 20 May 2021).