Adham Abou Ali, Othman A Malak, Karim Salem, George Alkhoury, Natalie Sridharan, Rabih A Chaer, Efthymios D Avgerinos
|
Abstract Extension of an iliofemoral thrombosis into the inferior vena cava (IVC), or from the IVC descending into the iliofemoral segments, can confer significant morbidity and mortality. Interventional management of acute deep vein thrombosis (DVT) has been controversial, but there is little doubt that certain subpopulations benefit, such as those with symptomatic IVC thrombosis. When considering an intervention, caval involvement introduces technical difficulties due to its larger diameter, high thrombus burden, bilateral limb clot extension and need for dual access. The frequent coexistence of an IVC filter increases the complexity even more. This review summarises the current indications and treatment modalities available for the management of acute DVT involving the vena cava. Keywords: Inferior vena cava, deep vein thrombosis, thrombectomy, thrombolysis, post-thrombotic syndrome Disclosure: EA is a consultant and member of the speakers bureau for Boston Scientific, Angiodynamics, BD Medical and INARI Medical. RAC is a consultant for Boston Scientific. All other authors have no conflicts of interest to declare. Received: 02 August Accepted: 15 November 2021Published online: 16 May 2022 Citation: Vascular & Endovascular Review 2022;5:e04. DOI:https://doi.org/10.15420/ver.2021.08 Correspondence Details: Efthymios Avgerinos, Division of Vascular Surgery, Heart and Vascular Institute, South Tower, Office 351.1, Presbyterian University Hospital, 200 Lothrop St, Pittsburgh, PA 15213, US. E: efavgerinos@gmail.com
Open Access:
This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
|
The interventional treatment of lower extremity DVT has rapidly evolved over the past decade, and despite existing controversy there is little doubt of its benefits in carefully selected patients.7 Catheter-directed interventions for acute iliofemoral DVT aim at rapid thrombus removal, maintenance of venous valve function and ultimately the lowering of PTS rates and improvement of quality of life. When considering an intervention, caval involvement introduces technical difficulties due to its larger diameter, high thrombus burden, bilateral limb clot extension and need for dual access. The frequent coexistence of an IVC filter increases the complexity even more. The purpose of this review is to summarise the current indications and treatment modalities available for the management of acute DVT with ascending (or descending) caval thrombosis.
Intervention for Acute Deep Vein Thrombosis Involving the Inferior Vena Cava
Catheter-directed interventions focus on early (and ideally) complete thrombus removal to rapidly improve symptomatology and reduce PTS incidence.7 Three major randomised controlled clinical trials and several institutional series have evaluated the utility of catheter-directed interventions compared with anticoagulation alone for the management of lower extremity DVT.8–10 The results have been conflicting, mainly due to improper patient inclusion or technical inappropriateness.11,12 Regardless of this, there is little doubt that certain subpopulations benefit, and the subpopulation with symptomatic IVC thrombosis is probably one of them. Although few studies have specifically addressed IVC involvement it seems that the more proximal and more extensive the thrombus, the higher the chance that the patient has severe symptoms and maximum benefit from an intervention. 13
A meta-analysis of the randomised trials published in the most recent European venous thrombosis guidelines reported that “early thrombus removal techniques are more effective than anticoagulation alone in preventing any PTS (RR 0.67; 95% CI [0.45–1.00]; p=0.05) and particularly moderate to severe PTS (RR 0.59; 95% CI [0.44–0.80]; p<0.001)” at the expense of an increased risk of bleeding with interventional therapy (RR 5.68; 95% CI [1.27–25.33]; p=0.02).14 However, considering that current practice is gradually shifting towards non- (or minimal) thrombolytic techniques this risk–benefit ratio will probably improve further. The European guidelines advocate the consideration of early thrombus removal strategies for selected patients with symptomatic iliofemoral DVT. The choice of therapy is left to the discretion of the treating physician.14
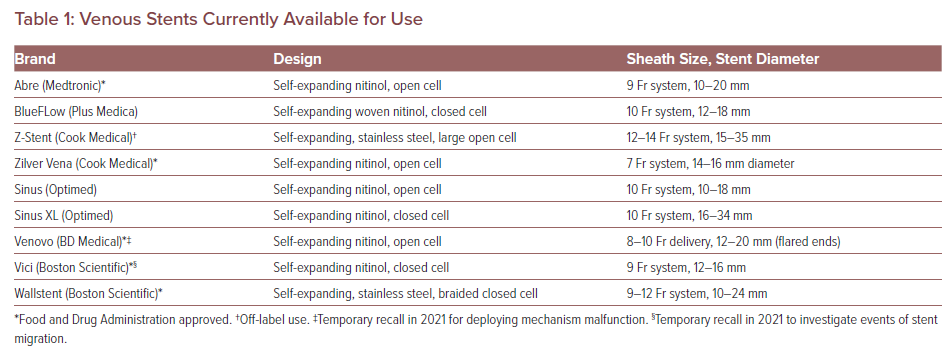
Acute Iliofemoral or Caval DVT Treatment Algorithm
Our current patient selection algorithm has been previously described.15 It is our practice to consider interventions for patients with iliofemoral DVT who have had symptoms for less than 30 days. Symptom severity and bleeding risks factor in the final decision on who and how to intervene (Figure 1).
Treatment Modalities
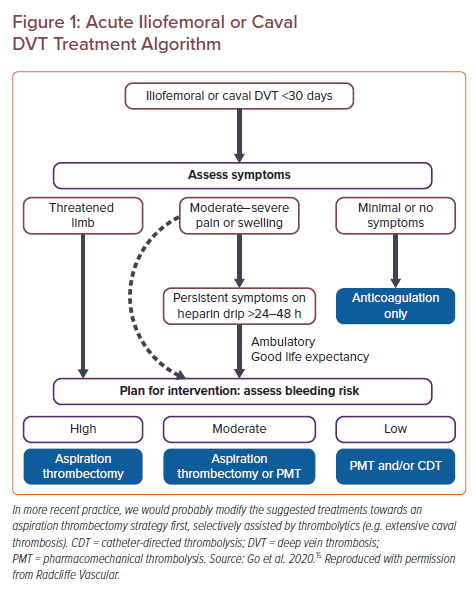
Anticoagulation is the standard treatment for DVT including caval thrombus extension. However, medical treatment alone in patients with complete iliocaval thrombosis involvement has been shown to be minimally effective. The interventional alternatives are summarised below (Figure 2).
Thrombolytic Techniques
Catheter-directed thrombolysis (CDT) involves the slow infusion of a plasminogen activator directly into the thrombus at a rate of 1 mg/h through a multi-sidehole infusion catheter. These catheters are the Unifuse (Angiodynamics), the Cragg-McNamara (Medtronic), and the Fountain (Merritt Inc.). The procedure is performed through a 5 Fr system via either a femoral or popliteal vein access; some interventionalists might opt for small saphenous or even proximal posterior tibial vein access. The patient is monitored in an intensive care unit throughout the infusion duration with complete blood counts and fibrinogen levels checked every 6 hours. The patient is eventually brought back for lysis termination and stenting after 8–24 hours. This technique can take up to 48 hours and multiple operating room trips to achieve complete thrombus resolution. The addition of ultrasound energy to CDT (EKOS, Boston Scientific) has not been translated into a clinically significant benefit over standard CDT.16 Technical success rates for CDT alone have ranged between 83% and 100%, with bleeding complications ranging between 4% and 9%.6,15–17
The associated bleeding risks, prolonged infusion time, and intensive care unit requirements have rendered thrombolytic techniques less appealing, especially since the advent of new-generation thrombectomy devices. Short-duration catheter thrombolysis, however, can soften thrombus and optimise subsequent aspiration thrombectomy. Particularly in extensive caval thrombosis, when there is no contraindication for lytics, it is the authors’ preference to prime thrombus with ~10 mg of tissue plasminogen activator (tPA; 2–4 mg on table followed by 1 mg/h for 6–8 hours). This will allow a less aggressive thromboaspiration and prevent blood loss (by aspiration) or even kidney injury when pharmacomechanical thrombectomy using AngioJet (Boston Scientific) is considered.18
On-table infusion of lytics inside the large mass of thrombus within the IVC can be more effective if done using small 3 ml syringes. This will enable a more efficient hand injection of the lytic solution (e.g. 4 mg tPA diluted in 20 ml heparinised saline).
A novel thrombolytic catheter is the Bashir Endovascular catheter (BEC, Thrombolex) that enables multichannel infusion in a basket configuration.19 Once deployed within the thrombus, the nitinol-reinforced basket at the tip of this catheter expands up to 45 mm, which can then be collapsed and redeployed to increase the surface area and binding sites for tPA within the thrombus. Its effectiveness is currently being trialled in the US.
Rheolysis – AngioJet
The rationale for pharmacomechanical thrombolysis (PMT) as a thrombus removal strategy has become more attractive given that PMT reduces the time required for thrombectomy to shorter and frequently single-session interventions.20 This reduces bleeding complications, intensive care unit stay and the associated costs.
PMT commonly involves a power pulse spray technique using the 8 Fr AngioJet Zelante catheter (Boston Scientific) that disperses thrombolytics forcefully along the thrombus (typically a 50–100 ml saline with 6–20 mg tPA solution). After allowing the thrombolytics to diffuse into the clot for 30 minutes, the AngioJet is switched to its rheolytic thrombectomy mode for clot removal. The unit/pump generates high-pressure pulsatile saline flow that exits the catheter tip through multiple retrograde-directed jets. These jets create a localised low-pressure zone (Bernoulli effect) for thrombus maceration and aspiration.
The technical success rate for PMT interventions for acute iliocaval thrombosis specifically ranges between 64% and 96%.20–22 One study that analysed 54 patients receiving PMT for IVC thrombosis noted a 64% primary technical success rate but a 100% stent-assisted success rate. The complication rate was 3.7%, with one patient requiring an intervention for a pulmonary embolism and another requiring a transfusion.21 In our institutional experience of 46 patients with caval thrombosis the technical success rate was 89.3% and the complication rate was 2.2%, with one access site haematoma requiring re-intervention. Compared with iliofemoral DVTs with no caval involvement, there were no differences in technical success, 30-day recurrence, or long-term patency rates in the caval involvement group. However, it was shown that IVC filter-associated caval thrombosis was less likely to respond to thrombolysis. Interestingly, caval extension of the DVT was associated with improved PTS outcomes compared with non-caval thrombosis.13
Available Systems for Pharmacomechanical or Purely Mechanical Caval Thrombectomy
AngioJet use has been associated with acute kidney injury in around 20% of patients, although the vast majority of cases are transient.22 Our institutional experience confirms the high likelihood of acute kidney injury when the IVC is involved. It is our recommendation that in the presence of caval thrombosis a two-stage technique maybe a safer practice, starting with CDT (if no contraindications) to reduce thrombus burden, and finalising at a second stage with PMT.18 Physicians should otherwise be careful to monitor the duration of time spent and the volume removed.
Non-thrombolytic Techniques
Over the past 5 years, novel percutaneous venous thrombectomy systems have been on the rise and we have seen an enormous practice shift following the evolution of these technologies.23 The devices are becoming larger, more powerful and smarter (i.e. incorporating sensors to detect blood loss), and can minimise, if not eliminate, lytics and their associated risks. Given their size, their efficacy in caval thrombus clearance is enhanced. There are no comparative trials or long-term data with regards to the mechanical thrombectomy techniques summarised in the following section. Their use in the hands of expert operators at high-volume centres remains the safest way to obtain more evidence about their potential future role in the management of caval thrombosis.
ClotTriever and FlowTriever
The ClotTriever device uses a 13 Fr or 16 Fr system through which the ClotTriever catheter, consisting of a coring element and a braided nitinol collection bag, is inserted and then deployed in the IVC above the thrombus. As the catheter is withdrawn, the thrombus is captured in the collection bag. Three to four passes of the device enable the largest amount of thrombus retrieval. The CLOUT registry is currently compiling a list of acute and chronic lower extremity DVT cases that use the ClotTriever catheter. Preliminary results indicate that more than three-quarters of patients have near-complete clot resolution, defined as >75% thrombus clearance. No device-related major adverse events or any major bleeding events were recorded.24 It is worth noticing that the presence of an IVC filter requires more complex interventional manoeuvres (e.g. internal jugular vein sheath access and ClotTriever wire snaring above the filter), which makes it cumbersome to use and should instead be avoided.
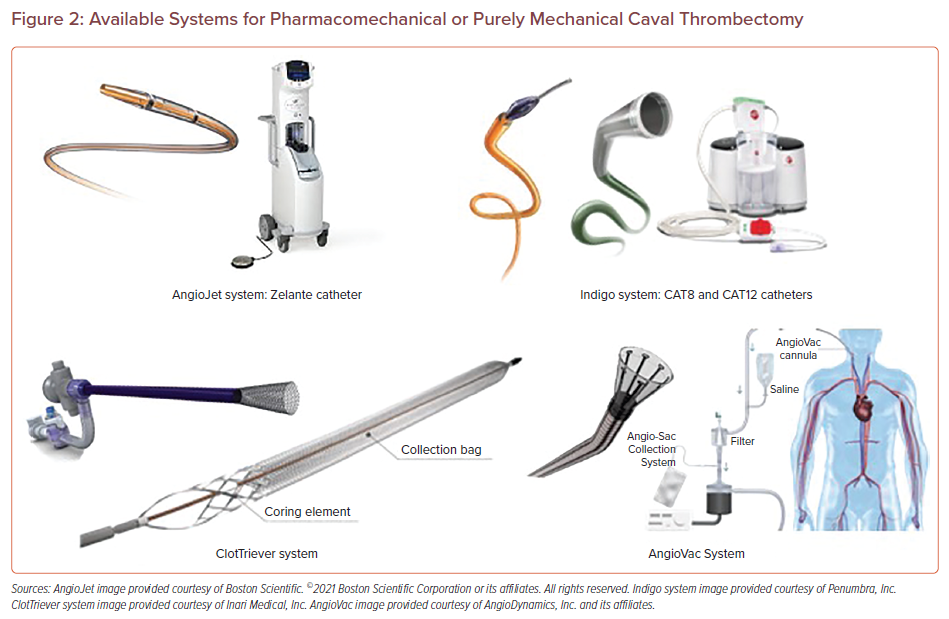
The FlowTriever system, typically used for caval thrombus, consists of a 16–24 Fr aspiration catheter. The aspiration catheter generates powerful suction via an attached vacuum-generating 60 ml custom-made syringe. Its large size makes it effective in removing large amounts of clot, but popliteal access can be challenging and potentially traumatic (Figure 3).
A retrospective review of 15 patients with caval thrombus treated with the ClotTriever and/or FlowTriever systems (used either separately or in combination) reported a technical success rate of 86.6% (13 of 15 patients) without the use of thrombolytics.25 There were no major bleeding events or any intensive care unit hospital stays; the median length of stay was 3 days.
The choice between the two devices is at the discretion of the operator; the ClotTriever’s ability to core out thrombus densely adherent to the vessel wall makes it a compelling option to remove the associated iliofemoral clot, whereas the FlowTriever’s large-bore catheter and powerful aspiration mechanism make it more suitable for extracting caval thrombus.
Persistent Right Leg Swelling and Failed Lyse Attempt Due to ‘Non-existent’ IVC in a 42-year-old Man
Indigo System CAT8 and Lightning 12
The Indigo CAT system consists of a catheter, a separator and a vacuum pump. The catheters are available in a variety of sizes from 3.4 Fr to 12 Fr. The CAT12 catheter is the most recent addition and is tailored towards large-bore vessels such as the IVC with or without the presence of a filter. The angled tip of these catheters in addition to the associated separator allows for thrombus fragmentation and clearing. The pump traditionally involved a manually controlled continuous suction.
The new Lightning 12 Intelligent Aspiration system has a dual pressure sensor that detects and differentiates thrombus from blood. Negative suction will be maintained as long as the catheter is in thrombus; once blood or continuous flow is detected, the suction becomes intermittent, thereby mitigating some of the blood loss associated with the earlier suction thrombectomy pumps. In a contemporary analysis of the CAT8 catheter a 60% technical success rate for iliofemoral DVTs was noted.26 The larger CAT12 system has recently gained approval for peripheral venous and pulmonary embolism treatment and is anticipated to offer significant advantages and efficiency over the smaller CAT8 (Figure 4).
AngioVac
The AngioVac (AngioDynamics) aspiration system comes with a 24 Fr suction cannula that is part of an extracorporeal veno-venous circuit that filters the blood and returns it via an 18 Fr reinfusion cannula at a separate access site. It is a very powerful device specifically designed to remove large clots in an en bloc fashion from large vasculature such as the IVC and/or the proximal iliac vessels, and it is otherwise too large to navigate in the femoropopliteal vessels.
The procedure needs to be done under general anaesthesia. The aspiration cannula should be typically introduced through a right internal jugular access to prevent proximal embolisation and pulmonary embolism. Once the cannula is placed across the thrombus, its self-expanding funnel-tip is deployed, opening up to 48 Fr, the extracorporeal circulation is initiated and slowly increased to a maximum rate of 3 l/min; the catheter is repeatedly advanced and withdrawn. A recent report demonstrated its utility in patients with caval thrombosis (9 of 16 patients), with complete thrombus extraction in 81.3% of the patients.27
Angiodynamics recently launched the AlphaVac System, an off-circuit, multi-purpose mechanical aspiration thrombectomy device. The AlphaVac System incorporates a new mechanical aspiration handle, and the extracorporeal circuit function will remain as optional.
Other Devices
Several other thrombectomy catheters older or new are available in the market but are either not well investigated or are not suited for the IVC: the Arrow-Trerotola (Teleflex Inc.), the Clot Buster Amplatz Thrombectomy Device (Microvena Corp.), the Cleaner (Argon Medical Devices Inc.), the Aspirex (BD Medical), the JETi8 (Walk Vascular), the Quick Clear (Philips) and the ReVene Thrombectomy catheter (Vetex Medical).
Iliocaval Stenting
Residual thrombus, either from incomplete lysis or aspiration thrombectomy, is considered a chronic component, and if untreated it is associated with an early recurrence and a more severe PTS development.7,28 As a result, the liberal use of stents is favoured to cover residual thrombus and any uncovered external compression (e.g. May–Thurner syndrome).29 The 2- and 5-year stent patency rates (primarily for Wallstent [Boston Scientific]) range between 65% and 95%.29 Our own iliac vein stenting experience demonstrated 3-year primary and secondary patency rates of 75.2% and 82.2%, respectively.7 Iliocaval stenting patency rates have been reported to be even higher.29 The rates are otherwise favourable, provided that the appropriate technique has been used (e.g. correct sizing and the stent landing on healthy venous segments with appropriate inflow). The advent of dedicated venous stents is changing the landscape of venous interventions in terms of ease of use, length and radial force, however, long-term results are lacking (Table 1).
Acute Bilateral Leg Swelling and Back Pain Diagnosed with Iliocaval Thrombosis Related to an IVC Filter Placed 10 years Previously in a 47-year-old Man
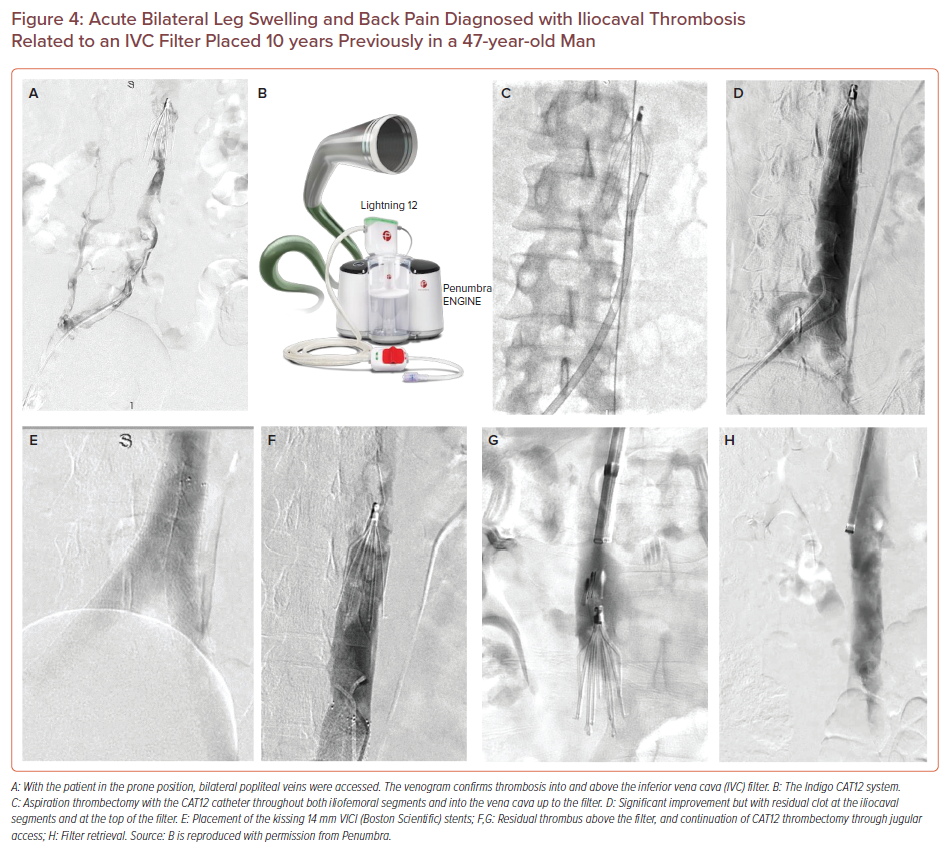
Although an isolated caval lesion may be easy to treat with a single large stent, it is not that frequent. Typically, the iliocaval bifurcation is involved and although multiple techniques have been described, the kissing stents technique seems to be popular, with very good long-term patency rates.30,31 When kissing stents are positioned they should be levelled at the same height within the IVC or else the one may be competing over the other.
For the few occasions in which an isolated caval stent, or extension above the renal veins, is required there are only two stainless steel stents available in the US and one more in Europe. The Wallstent (Boston Scientific) and the Z-stent (Cook) are available in large enough sizes (>20 mm) for IVC stenting (up to 24 mm and 35 mm, respectively). The Wallstent is strong and flexible but deployment is inaccurate due to foreshortening. The Z-stent offers a few advantages over the Wallstent due to its minimal foreshortening, greater radial force and its larger interstices, but it has large fixing spines and therefore carries a higher risk of caval perforation.32,33
The presence of a filter is highly debated with regard to whether it should be removed or over-stented. Few centres have documented expertise in safely removing chronically thrombosed filters, and a lot of complications related to attempted retrievals are probably underreported.34 Stent placement for chronically thrombosed IVC filters has previously been described by numerous authors, and although it has been shown to be technically feasible and safe in various series, large studies with long-term follow-up are lacking.35–38
It is our preference to attempt to remove the filter immediately after the thrombectomy (or in the same admission), but if excessive interventional manoeuvres are required, stenting across is a reasonable approach.
Intravascular Ultrasound
Intravascular ultrasound (Philips) enables the acquisition of detailed images in an axial plane relative to the catheter tip. Intravascular ultrasound has been shown in multiple studies to be superior for accurate lesion identification compared with plain venography.39
Specifically, for caval thrombosis, intravascular ultrasound can provide more accurate data on thrombus burden, IVC filter positioning and clot around it as well as the renal vein orifices and renal vein thrombosis. It is also essential to guide stent diameter, landing zones and to confirm a satisfactory final outcome. Despite that, one recent survey on iliocaval stent reconstruction reported that only 64.6% of operators used intravascular ultrasound to guide reconstruction, a testament to the persistent inconsistencies in venous thrombosis management.40
Prophylactic Inferior Vena Cava Filters
A theoretical risk factor for thrombolysis or aspiration thrombectomy is iatrogenic pulmonary embolism related to the instrumentation of extensive amounts of fresh thrombus. Although a small, randomised trial has indicated a higher rate of clinically significant pulmonary embolism in patients not receiving an IVC filter, there was no mortality difference and subsequent contemporary studies recommended highly selective IVC filtration.41 Pulmonary emboli can be unavoidable, but they are rarely clinically meaningful for otherwise low-risk patients, and placement of an IVC filter may introduce complexity and other potential risks.
In our experience, IVC filters are rarely used, irrespective of the type of catheter intervention. Patients who might benefit are those with clinically significant pulmonary embolism on presentation or known low cardiopulmonary reserve.42 If a filter is used, it should be placed prior to the intervention through the internal jugular vein or contralateral femoral vein,and retrieved at the end of the procedure or at any time before discharge, provided that anticoagulation is maintained.
Anticoagulation
All patients with a DVT diagnosis should retain their therapeutic anticoagulation in preparation for and during the procedure. Unfractionated or low-molecular-weight heparin can be used. Maintenance of therapeutic anticoagulation during ongoing thrombolysis varies with institutional policies.
In our practice, for DVT lysis we maintain small heparin doses through the sheath. The patient is fully heparinised in aspiration thrombectomy procedures. Postoperative anticoagulation, particularly when stents have been placed, should include low-molecular-weight heparin for 4–6 weeks and probably lifelong anticoagulation thereafter (given the extent of the caval thrombotic event and the reconstruction).
The role of antiplatelet agents is highly debated and practices vary.
Conclusion
Caval thrombus extension is an increasingly encountered pathology especially with the continued use of IVC filters. Treating iliocaval thrombosis with an interventional approach incorporating novel thrombectomy techniques and selective use of thrombolytics is gaining popularity as a means of immediate and long-term morbidity and PTS reduction. An appropriate technique for maximal thrombus elimination and liberal stenting between healthy segments are the cornerstone of a successful long-term outcome.
-
-
- Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost 2008;6:1105–12.
Crossref| PubMed - Grewal S, Chamarthy MR, Kalva SP. Complications of inferior vena cava filters. Cardiovasc Diagn Ther 2016;6:632–41.
Crossref| PubMed - Milovanovic L, Kennedy SA, Midia M. Procedural and indwelling complications with inferior vena cava filters: frequency, etiology, and management. Semin Intervent Radiol 2015;32:34–41.
Crossref| PubMed - Kahn SR. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 2008;149:698–707.
Crossref| PubMed - Shah NG, Wible BC, Paulisin JA, et al. Management of inferior vena cava thrombosis with the FlowTriever and ClotTriever systems. J Vasc Surg Venous Lymphat Disord 2021;9:615–20.
Crossref| PubMed - Alkhouli M, Zack CJ, Zhao H, et al. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation versus anticoagulation alone in the treatment of inferior vena caval thrombosis. Circ Cardiovasc Interv 2015;8:e001882.
Crossref| PubMed - Avgerinos ED, Saadeddin Z, Abou Ali AN, et al. Outcomes and predictors of failure of iliac vein stenting after catheter-directed thrombolysis for acute iliofemoral thrombosis. J Vasc Surg Venous Lymphat Disord 2019;7:153–61.
Crossref| PubMed - Enden T, Haig Y, Kløw N-E, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379:31–8.
Crossref| PubMed - Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med 2017;377:2240–52.
Crossref| PubMed - Notten P, Ten Cate-Hoek AJ, Arnoldussen C, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol 2020;7:40–9.
Crossref| PubMed - Avgerinos ED, Chaer RA. The ATTRACTiveness of catheter-directed thrombolysis. J Vasc Surg Venous Lymphat Disord 2018;6:303.
Crossref| PubMed - Avgerinos ED, Jalaie H, Chaer RA. Catheter interventions: an unresolved clinical controversy. Lancet Haematol 2020;7:e189.
Crossref| PubMed - Avgerinos ED, El-Shazly O, Jeyabalan G, et al. Impact of inferior vena cava thrombus extension on thrombolysis for acute iliofemoral thrombosis. J Vasc Surg Venous Lymphat Disord 2016;4:385–91.
Crossref| PubMed - Kakkos SK, Gohel M, Baekgaard N, et al. European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg 2021;61:9–82.
Crossref| PubMed - Go C, Chaer RA, Avgerinos ED. Catheter interventions for acute deep venous thrombosis: who, when and how. Vasc Endovasc Rev 2020;3:e07.
Crossref - Engelberger RP, Stuck A, Spirk D, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis: 1-year follow-up data of a randomized-controlled trial. J Thromb Haemost 2017;15:1351–60.
Crossref| PubMed - Comerota AJ, Kearon C, Gu CS, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation 2019;139:1162–73.
Crossref| PubMed - Salem KM, Saadeddin Z, Malak OA, et al. Risk factors for acute kidney injury after pharmacomechanical thrombolysis for acute deep vein thrombosis. J Vasc Surg Venous Lymphat Disord 2021;9:868–73.
Crossref| PubMed - Al-Otaibi M, Iftikhar O, Brailovsky Y, et al. Catheter-directed thrombolysis of iliocaval thrombosis in patients with COVID-19 infection. JACC Case Rep 2020;2:2016–20.
Crossref| PubMed - Go C, Saadeddin Z, Pandya Y, et al. Single- versus multiple-stage catheter-directed thrombolysis for acute iliofemoral deep venous thrombosis does not have an impact on iliac vein stent length or patency rates. J Vasc Surg Venous Lymphat Disord 2019;7:781–8.
Crossref| PubMed - Ye K, Qin J, Yin M, et al. Outcomes of pharmacomechanical catheter-directed thrombolysis for acute and subacute inferior vena cava thrombosis: a retrospective evaluation in a single institution. Eur J Vasc Endovasc Surg 2017;54:504–12.
Crossref| PubMed - Morrow KL, Kim AH, Plato SA, et al. Increased risk of renal dysfunction with percutaneous mechanical thrombectomy compared with catheter-directed thrombolysis. J Vasc Surg 2017;65:1460–6.
Crossref| PubMed - Wang W, Sun R, Chen Y, Liu C. Meta-analysis and systematic review of percutaneous mechanical thrombectomy for lower extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord 2018;6:788–800.
Crossref| PubMed - Dexter DJ. First look at CLOUT registry reveals over 75% of DVT patients have near-complete clot resolution with ClotTriever. Venous News 17 November 2019. https://venousnews.com/first-clout-registry-data-reveal-75-of-dvt-patients-resolve-75-of-clot-with-mechanical-thrombectomy (accessed 27 November 2019).
- Shah NG, Wible BC, Paulisin JA. Management of inferior vena cava thrombosis with the FlowTriever and ClotTriever systems. J Vasc Surg Venous Lymphat Disord 2021;9:615–20.
Crossref| PubMed - Lopez R, DeMartino R, Fleming M, et al. Aspiration thrombectomy for acute iliofemoral or central deep venous thrombosis. J Vasc Surg Venous Lymphat Disord 2019;7:162–8.
Crossref| PubMed - Rajput FA, Du L, Woods M, Jacobson K. Percutaneous vacuum-assisted thrombectomy using AngioVac aspiration system. Cardiovasc Revasc Med 2020;21:489–93.
Crossref| PubMed - Haig Y, Enden T, Slagsvold CE, et al. Determinants of early and long-term efficacy of catheter-directed thrombolysis in proximal deep vein thrombosis. J Vasc Interv Radiol 2013;24:17–24.
Crossref| PubMed - Mahnken AH, Thomson K, de Haan M, et al. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol 2014;37:889–97.
Crossref| PubMed - Neglén P, Darcey R, Olivier J. Bilateral stenting at the iliocaval confluence. J Vasc Surg 2010;51:1457–66.
Crossref| PubMed - Neglén P. Stenting is the “method-of-choice” to treat iliofemoral venous outflow obstruction. J Endovasc Ther 2009;16:492–3.
Crossref| PubMed - Funaki B. Inferior vena caval stenting. Semin Intervent Radiol 2004;21:347–9.
Crossref| PubMed - Alkhouli M, Morad M, Narins CR, et al. Inferior vena cava thrombosis. JACC Cardiovasc Interv 2016;9:629–43.
Crossref| PubMed - Desai KR, Xiao N, Karp J, et al. Single-session inferior vena cava filter removal, recanalization, and endovenous reconstruction for chronic iliocaval thrombosis. J Vasc Surg Venous Lymphat Disord 2019;7:176–83.
Crossref| PubMed - Chick JFB, Jo A, Meadows JM, et al. Endovascular iliocaval stent reconstruction for inferior vena cava filter-associated iliocaval thrombosis: approach, technical success, safety, and two-year outcomes in 120 patients. J Vasc Interv Radiol 2017;28:933–9.
Crossref| PubMed - Ye K, Lu X, Li W, et al. Outcomes of stent placement for chronic occlusion of a filter-bearing inferior vena cava in patients with severe post-thrombotic syndrome. Eur J Vasc Endovasc Surg 2016;52:839–46.
Crossref| PubMed - Neglén P, Oglesbee M, Olivier J, et al. Stenting of chronically obstructed inferior vena cava filters. J Vasc Surg 2011;54:153–61.
Crossref| PubMed - Arabi M, Krishnamurthy V, Cwikiel W, et al. Endovascular treatment of thrombosed inferior vena cava filters: techniques and short-term outcomes. Indian J Radiol Imaging 2015;25:233–8.
Crossref| PubMed - Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord 2017;5:678–87.
Crossref| PubMed - Hage AN, Srinivasa RN, Abramowitz SD, et al. Endovascular iliocaval stent reconstruction for iliocaval thrombosis: a multi-institutional international practice pattern survey. Ann Vasc Surg 2018;49:64–74.
Crossref| PubMed - Sharifi M, Bay C, Skrocki L. Role of IVC filters in endovenous therapy for deep venous thrombosis: the FILTER-PEVI (Filter Implantation to Lower Thromboembolic Risk in Percutaneous Endovenous Intervention) trial. Cardiovasc Intervent Radiol 2012;35:1408–13.
Crossref| PubMed - Avgerinos ED, Hager ES, Jeyabaln G, et al. Inferior vena cava filter placement during thrombolysis for acute iliofemoral deep venous thrombosis. J Vasc Surg Venous Lymphat Disord 2014;2:274–81.
Crossref| PubMed
- Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost 2008;6:1105–12.
-